Alamy Stock Photo
Safety features mandated through the falsified medicines directive (FMD) have not detected any falsified medicines in the UK since its launch, The Pharmaceutical Journal has learned.
Data published by SecurMed, the UK national medicines verification organisation, shows that as of 4 December 2019; approximately 44.7 million medicine packs have been dispensed through the UK national medicines verification system, since the FMD went live on 9 February 2019.
A spokesperson from the Medicines and Healthcare products Regulatory Agency (MHRA) told The Pharmaceutical Journal that a small number of falsified medicines had been identified in the legal supply chain since the FMD’s launch.
However, they added that these were not picked up using the 2D barcodes featured on medicine packs as part of the FMD safety feature requirements.
“The MHRA has had no confirmed reports of falsified medicines being detected by scanning of 2D barcodes against the national medicines verification system,” the spokesperson said.
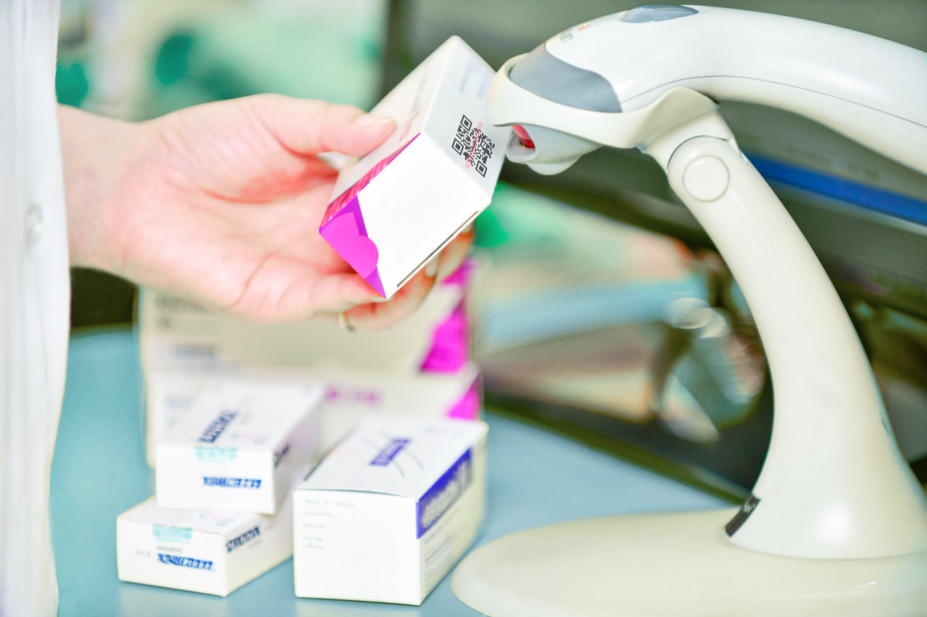
Under the requirements, packs of medicine are required to include a unique 2D barcode. The barcode enables each pack to be serialised with a unique randomised number, which is scanned and authenticated before dispensing.
Jerome Bertin, general manager of SecurMed, said the UK national medicines verification body was, alongside other national medicines verification bodies across Europe, “still dealing with a lot of noise from manufacturers and end users” as they get used to the requirements of medicines serialisation and FMD legislation.
“This is somewhat masking the signals of potential falsifications; though we do interact with manufacturers, end users and the MHRA to look at issues as they are raised,” Bertin said.
“I do not think this is surprising: we expected it to take some time for everyone to get used to the FMD requirements.”
If the UK leaves the EU with a deal, it is expected that the FMD will continue to apply in the UK during any transition period. The Conservative Party has said that they plan to take the UK out of the EU by the end of January 2020, and that the transition period will not be extended beyond December 2020.
The MHRA has previously said that in the event of a no-deal Brexit, the legal obligation to adhere to FMD regulations “would be removed for actors in the UK supply chain”, but that it would consider a UK-only version of the law.
In October 2019, SecurMed proposed that in the event of a no-deal Brexit; the UK should retain a degree of connectivity to the European Medicines Verification System (EMVS) “in the short term”, to prevent inappropriate system errors and alerts being triggered in the EMVS and other national databases.
You may also be interested in
GPhC writes to pharmacy teams after methotrexate dispensed with instruction to take once daily

Medicines commission calls for greater clarity on risk of suicidal behaviour from antidepressants
