Mediscan / Alamy Stock Photo
A Scottish health board has confirmed that it will stop doctors providing one medicine and review 14 others to ensure they are being provided in line with licensed indications.
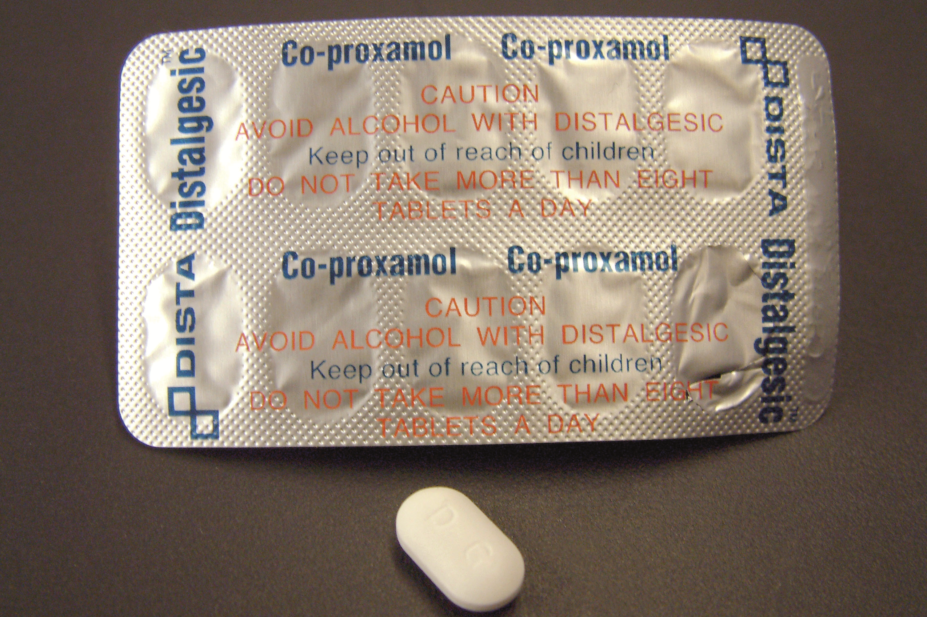
Speaking to The Pharmaceutical Journal, NHS Borders said that any patients currently receiving the analgesic co-proxamol will be reviewed by their GP and prescribed an alternative where appropriate.
A spokesperson said it is also currently reviewing a further 14 medicinal products to ensure that prescribing is in line with licensed indications and that the products are only used for indications recommended by the Scottish Medicines Consortium (SMC).
The products under review are buprenorphine patches; dosulepin; modified release doxazosin; ezetimibe; immediate release fentanyl; lidocaine plasters; liothyronine; lutein and antioxidants; rubifacients and poultices; once-daily tadalafil; travel vaccines; glucosamine and chondroitin, trimipramine and homeopathy.
The review was requested by NHS Borders’ GP Advisory Committee and endorsed by its Area Drug and Therapeutics Committee.
Of the medicines under review, 13 are among the 18 products identified by NHS England as items that should no longer be routinely prescribed in primary care as part of its guidance issued to clinical commissioning groups in November 2017.
In April 2018, another Scottish health board, NHS Tayside, was widely reported to be stopping one-off prescriptions for paracetamol and ibuprofen. But, on 3 October 2018, a spokesperson for NHS Tayside told The Pharmaceutical Journal that it had “no plans to stop prescribing ibuprofen or paracetamol in primary care where they are felt to be clinically appropriate”.
Asked if NHS Tayside would be reviewing other medicines currently available on NHS prescription, the spokesperson added: “We continually review our formulary based on the clinical evidence, safety and cost effectiveness of medicines.
“Decisions may be made to stop prescribing certain medicines as a result of changes to our formulary, new treatments becoming available or new evidence about the benefits of a drug.”