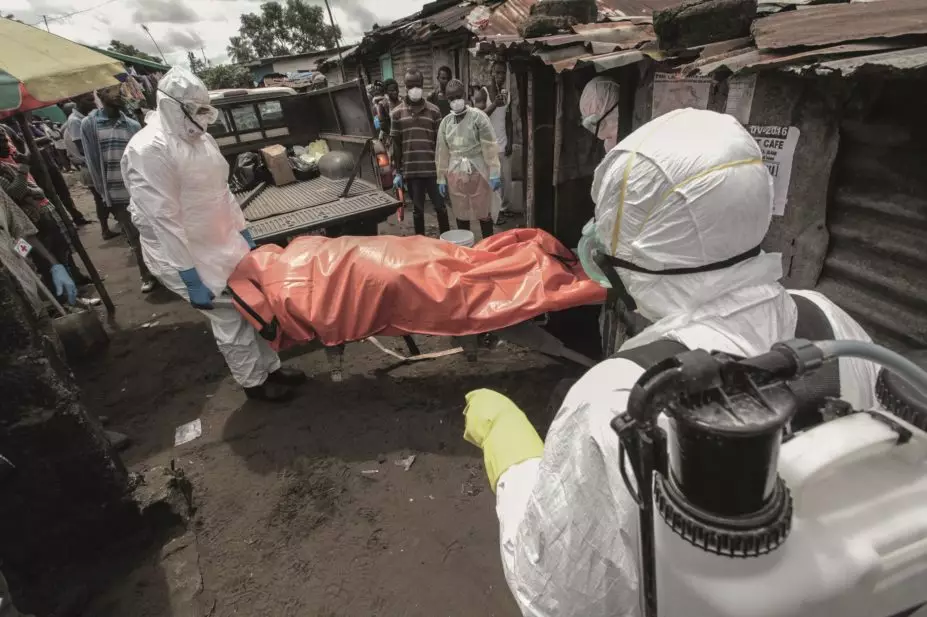
Marcus DiPaola / NurPhoto / Rex Features
Johnson & Johnson’s pharmaceuticals unit, Janssen, will invest US$200m in accelerating and expanding production of its Ebola vaccine.
Janssen says it aims to produce more than one million doses of the vaccine regimen in 2015, a quarter of a million of which are expected to be released for clinical trials by May 2015.
The company says it is collaborating with the World Health Organization (WHO) as well as the National Institute of Allergy and Infectious Diseases (NIAID). Its vaccine, which was discovered in a collaborative research programme with the National Institutes of Health (NIH), combines a Janssen preventive vaccine with a treatment from Bavarian Nordic, a biotech company based in Denmark.
This combination regimen has shown “promising results”, Janssen says, but only in pre-clinical studies. The company now plans to test it on humans for the first time. It consists of two vaccine components that are based on AdVac technology from Crucell Holland, which is part of Janssen, and the MVA-BN technology from Bavarian Nordic.
The current Ebola outbreak began in West Africa in early 2014 and has particularly affected Liberia, Sierra Leone and Guinea, with several cases emerging in Europe and the United States. The WHO estimates that around 5,000 people have died from the virus, with around 10,000 believed to have been infected. Past Ebola outbreaks have shown a mortality rate of 90%, but in this outbreak it appears to be around 50%.
There is currently no clinically-tested vaccine available for Ebola, but major pharmaceutical firms are racing to develop both products and clinical tests to detect and kill the virus. One of these is London-based GlaxoSmithKline, but the company admitted on 17 October 2014 that despite efforts to expedite the process, its vaccine will not be ready in time for use against the current outbreak.
Janssen, it seems, is more optimistic about its ability to deliver a medicine by 2015, although the WHO believes as many as 20,000 could have died from the virus by the end of 2014.
Doctors have been treating some Western patients with the experimental ZMapp, a medicine that has only been used in animal studies and is manufactured by San Diego-based biotech firm Mapp. The drug has delivered mixed results, however, and it is not known whether recovery of patients with the virus can be attributed to ZMapp.