Shutterstock.com
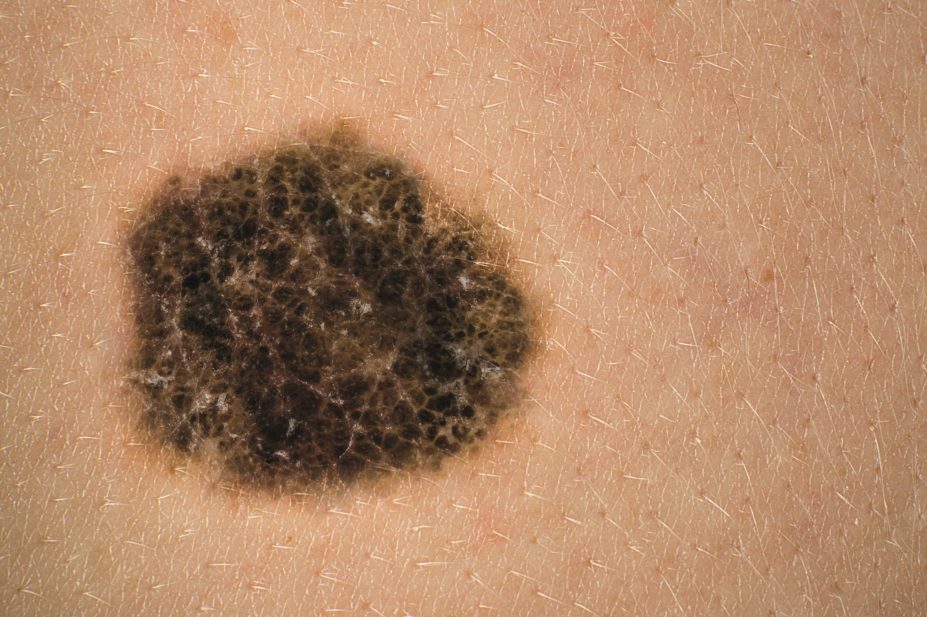
The National Institute for Health and Care Excellence (NICE) recommends in draft guidance that pembrolizumab (Keytruda) be made available on the NHS for the treatment of some patients with advanced melanoma.
The proposal comes just over a month after drug’s positive scientific opinion — which had made the product available to some skin cancer patients under the UK government’s Early Access to Medicines (EAMs) scheme — expired. Under the EAMS scheme, drugs in development that do not have a licence are made available when there is a high unmet clinical need. Pembrolizumab was the first drug to be approved under the scheme.
On 7 September 2015, NICE in its draft guidance recommends that pembrolizumab be made available to patients with melanoma in cases where the disease has progressed despite being treated with ipilimumab and, for BRAF V600 mutation-positive disease, a BRAF or MEK inhibitor.
Its availability, says NICE, is conditional on its manufacturer Merck Sharp & Dohme providing it at a discount in an agreed patient access scheme. Pembrolizumab is currently commercially available at the price of £1,315 per 50mg vial.
The NICE recommendation is out for consultation until 22 September 2015, with the final guidance expected to be published in October 2015.
You may also be interested in

Tackling the NHS drug budget: why we set up a regional collaboration for medicines value
Lack of joined-up working between pharmacy and general practice is ‘nonsensical’, says former BMA chair
