Science Photo Library
A new single-dose antibiotic could benefit patients, such as intravenous drug users, whose lifestyle makes it difficult to comply with a week-long course of vancomycin.
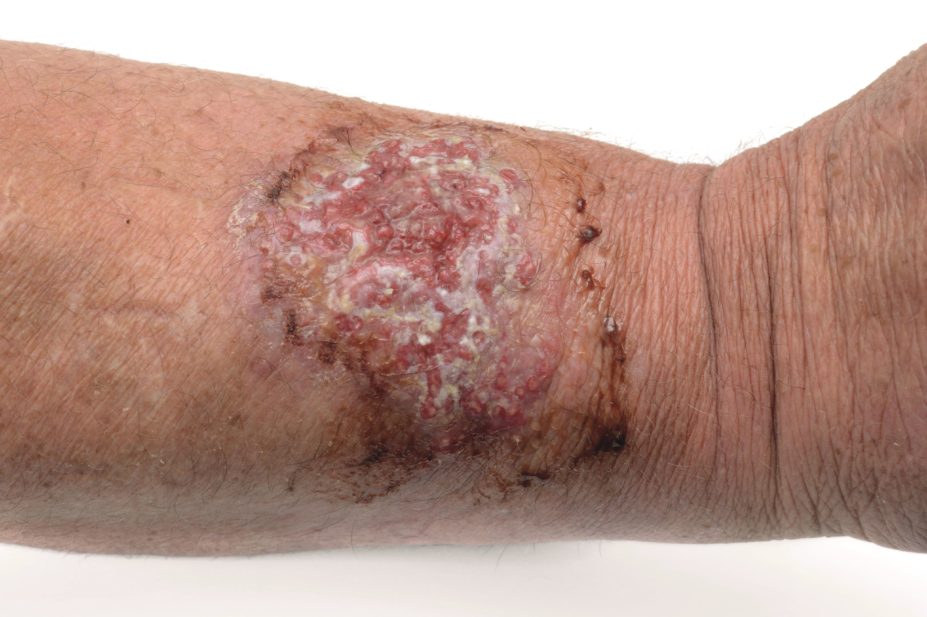
The new antibiotic, oritavancin, has just been approved by the US Food and Drug Administration, and in a trial of 787 patients with skin infections, it was no less effective than vancomycin.
The ease of a single-dose regimen will offer benefits to a patient population for whom compliance may be a real issue, according to Ken LaPensee, a health economist at The Medicines Company in Parsippany, New Jersey. “Not all patients come back for follow-up treatment, especially people who might have behavioral issues,” he explained.
Oritavancin was approved in the US in August and a decision on a European application is expected in the first half of 2015, said LaPensee, lead author on a poster presented on 8 September 2014 at the annual American Society for Microbiology’s ICAAC meeting in Washington, DC[1]
.
LaPensee’s poster revealed data from the SOLO I and II trials, comparing oritavancin against vancomycin in patients with acute skin infections. The study was a global trial and included patients in outpatient settings, of whom more than 50% were intravenous drug users.
“The average length of stay for patients treated with vancomycin is about four days, and almost nobody finishes the entire course of drug while an in-patient. They need follow-on treatment, which will take an additional three to seven days,” said LaPensee, noting that the course of treatment can be expensive.
LaPensee said that one single 1200mg dose of oritavancin is no less effective than seven to ten days of vancomycin. He said the ease and convenience of taking the treatment in one session would be especially beneficial to subgroups of the population with serious skin infections. Of the 787 patients described in the poster, 56% of those in the ambulatory subgroup in the oritavancin population, and 54.5% of those who received vancomycin, were intravenous drug users.
The primary outcome of the study was to stop spread of the infection, or a reduction in the size of the lesion, absence of fever, and no use of rescue antibiotics by a point in time between 48 and 72 hours from the start of treatment, according to the poster. The secondary endpoints included investigator-measured cure at 7 to 14 days following the start of treatment, and a reduction in the size of the lesion by 20% or more. Safety follow-up was conducted up to 60 days, the poster said.
The cure rate was similar between the two groups, at 83.9% in the oritavancin group and 81.8% among those given vancomycin. The two medicines showed the same efficacy, with oritavancin achieving the primary composite endpoint in 80.6% of patients, compared with 78.3% for vancomycin. Of those outpatients who received oritavancin, 65.2% experienced an adverse event, compared with 67.9% of those in the vancomycin group.
However, drug-related adverse effects occurred less frequently among the oritavancin patients (32.5%) than the vancomycin group (43.2%, confidence intervals not stated for adverse event data). Hypersensitivity in the oritavancin group was 13.0%, compared with 28.3% in those patients on vancomycin. Itchiness was experienced by 3.3% of patients on oritavancin, compared with 13.9% of the vancomycin patients.
Tachycardia occurred more frequently among patients taking oritavancin than it did among those taking vancomycin (4.9% versus 2%, confidence intervals not stated).
References
[1] LaPensee K, Fan W, Lodise T et al. Efficacy and safety of single dose oritavancin compared to 7-10 days of vancomycin in patients with acute bacterial skin and skin structure infections treated in the outpatient setting. [Poster L-1729] American Society for Microbiology. Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington DC. 8 September 2014.