Stephen C Dickson / Wikimedia Commons
Researchers at Cardiff University have developed a quantitative risk assessment tool to predict the likelihood of a drug either turning into a toxic version of itself, or losing efficacy, once it has entered the body[1]
.
The predictive tool, which was developed in collaboration with Liverpool John Moores University and AstraZeneca, enables the user to trawl through large databases of pharmaceutical drugs and assess the likelihood of a drug undergoing a process called ‘racemisation’, whereby the drug flips into a mirror image of itself and consequently becomes either inert, or harmful. This is thought to be the result of interaction between the drug and aqueous conditions in the body.
Racemisation, described by the researchers as a “cruel blind spot”, can result in wasted material and human resources, as well as potential harm to the user. It is hoped this new tool will lead to a significant reduction in the financial risk associated with drug development and increase the efficacy and safety of new drugs.
Drug compounds often exist in either a right- or left-handed form, each with an identical chemical composition but a structure that is a mirror image of each other and therefore non-superposable. These different forms of the same drug are known as enantiomers, meaning ‘opposite’, and often only one of the enantiomers is responsible for the desired effect while the other might be inactive or result in an adverse effect.
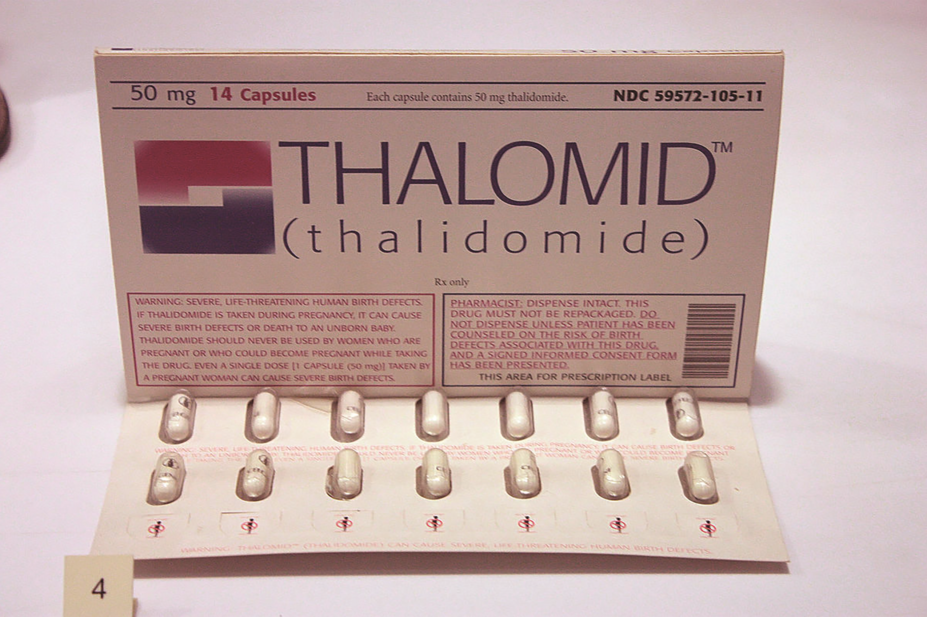
A famous example of this is thalidomide, first marketed in 1957, which was withdrawn from the market when it was found to cause birth defects. One enantiomer of the drug caused a sedative effect, as wanted, while the other led to birth defects.
“Following the thalidomide disaster, researchers worldwide have focussed on making compounds enantioselectively — that is containing just one enantiomer,” explained Niek Buurma, lead author of the study and a lecturer in physical organic chemistry at Cardiff University.
“However, while compounds are routinely tested to ensure they are inherently stable under physiological conditions, not much thought has been given to how to prevent configurational instability at the design stage, using suitable predictive models.”
Buurma said that he believed that the risk assessment would make it possible to manufacture safer medication by helping the pharmaceutical industry to quickly spot medication that will fail during development and focus their efforts on compounds that are more likely to work.
References
[1] Ballard A, Ahmad H, Narduolo S et al. Quantitative prediction of rate constants for aqueous racemization to avoid pointless steroselective syntheses. Angew Chem Int Ed 2017, 56, 1–5. doi: 10.1002/anie.201709163