CID / ISM / Science Photo Library
Pembrolizumab significantly extends the survival of patients with recurrent or metastatic head and neck squamous cell carcinoma, compared with the current standard of care, according to the results of a phase III trial published in The Lancet (30 November 2018)[1]
.
The study involved 495 patients with disease progression either during or after platinum-based chemotherapy, who were randomly assigned to pembrolizumab 200mg every three weeks or standard of care (which is methotrexate, docetaxel or cetuximab).
The researchers found that median overall survival was significantly longer in the pembrolizumab group at 8.4 months, compared with 6.9 months in the standard of care group. Sub analyses indicated that higher expression of PD-L1, the checkpoint inhibitor targeted by pembrolizumab, predicted a greater response to therapy.
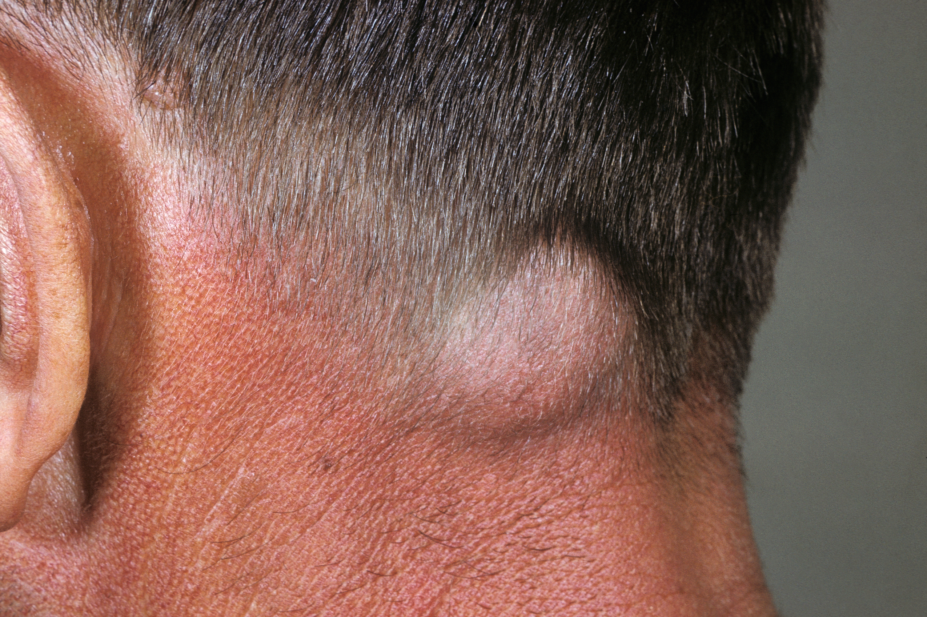
At least half of patients with this type of cancer develop recurrence or metastatic disease within three years of initial treatment, the researchers explained.
“The clinically meaningful prolongation of overall survival and favourable safety profile of pembrolizumab … support the further evaluation of pembrolizumab as a monotherapy and as part of combination therapy in earlier stages of disease,” the team concluded.
References
[1] Cohen W, Soulières D, Le Tourneau C et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase III study. Lancet 2018. doi: 10.1016/S0140-6736(18)31999-8