Wikidoc.org (Creative Commons Attribution/Share-Alike License)
Proton pump inhibitors (PPIs) have been linked with rare cases of subacute cutaneous lupus erythematosus (SCLE), according to a drug update issued by the Medicines and Healthcare products Regulatory Agency (MHRA) on 8 September 2015.
PPIs, which reduce gastric acid production, including esomeprazole, lansoprazole, omeprazole, pantoprazole and rabeprazole, are available on prescription to manage reflux oesophagitis, gastric and duodenal ulcers, and Zollinger-Ellison syndrome. Some PPIs are also available over the counter for self-management of heartburn and indigestion.
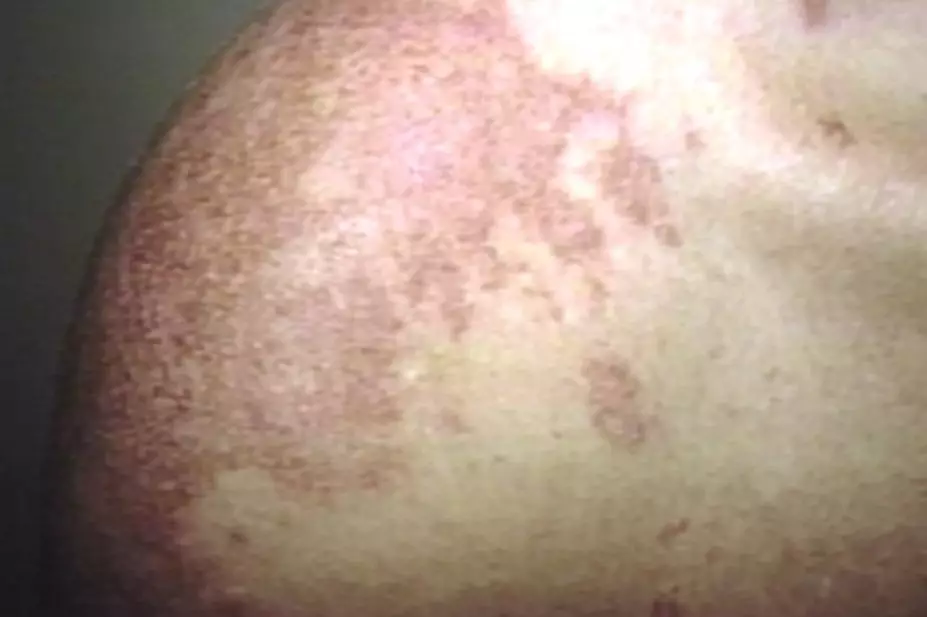
SCLE shows up as lesions on sun-exposed skin, and can be diagnosed by skin and blood tests. While only very few cases have been reported to regulatory bodies, including through the UK’s Yellow Card Scheme, case-control studies have shown that PPIs can increase the risk of developing SCLE almost three-fold compared with the general population. However, doctors and patients need to be aware that symptoms may appear weeks, months or even years after taking the drug, which could make spotting the connection harder.
If patients taking PPIs report scaly plaques or psoriasis-type skin lesions along with joint pain, healthcare professionals should advise them not to expose skin to sunlight, and suggest that they stop taking the PPI. SCLE symptoms generally resolve after stopping the PPI, but the side effect should be reported on a Yellow Card.