Bobjgalindo / Wikimedia Commons
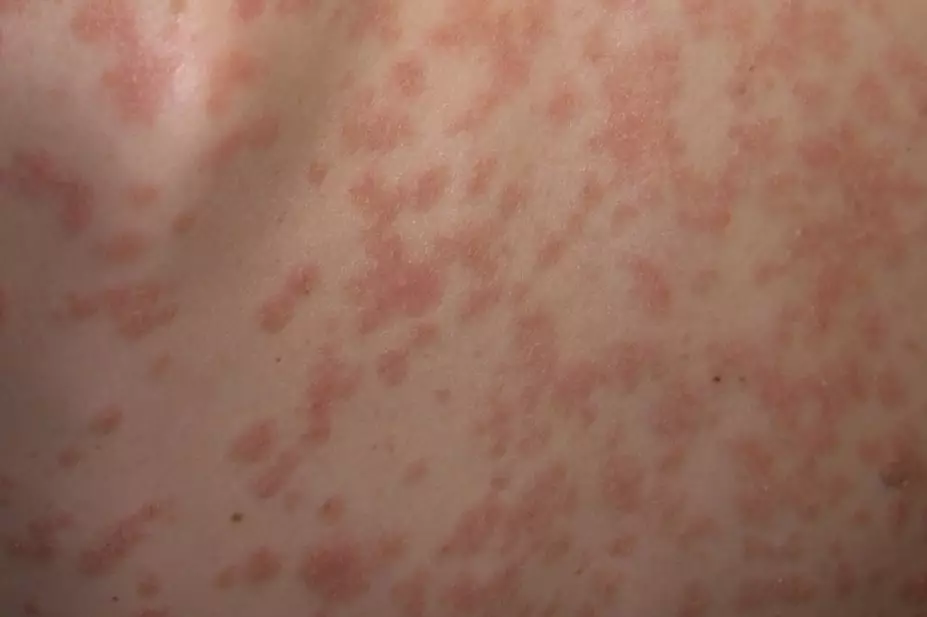
Ustekinumab, a monoclonal antibody (mAb) that binds the interleukin IL-12 subunit p40, which is shared by IL12 and IL23, is effective against psoriasis.
In a letter published in Nature (online, 9 March 2015)[1]
, Merck reports its phase I trial with the company’s new mAb, tildrakizumab, in 74 patients with moderate to severe psoriasis. By specifically targeting IL-23, the report says, tildrakizumab reduces psoriasis symptoms by 75%, and is safe and effective against the disease.
“The patients tolerated even the maximum doses of the mAb,” says Sauzanne Khalilieh, a scientist at Merck & Co. Kenilworth, New Jersey. Common side effects were headache, nasopharyngitis, upper respiratory infection and cough.
Merck has now started phase III trials and has entered into a licensing agreement with Sun Pharmaceutical Industries, who will acquire worldwide rights to tildrakizumab.
- This article was updated on 31 March 2015 to clarify that ustekinumab targets both IL12 and IL23.
References
[1] Kopp T, Riedl E, Bangert C et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature 2015. doi:10.1038/nature14175.