Science Photo Library
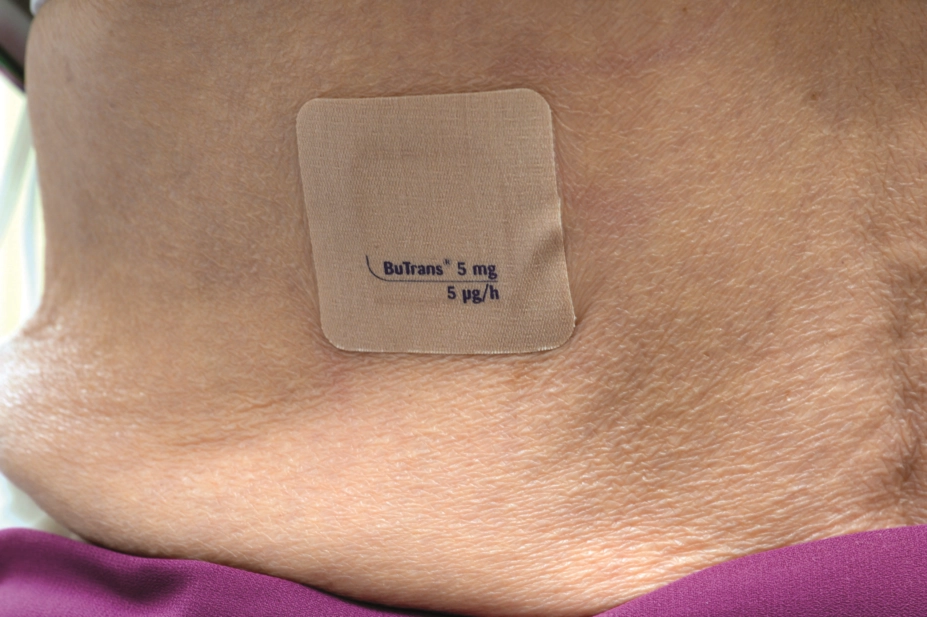
Research has found that more than half of people living with dementia who are treated with transdermal buprenorphine discontinue the drug because of adverse events[1]
.
The study involved 162 people living in nursing homes in Norway with advanced dementia and depression. They were randomly assigned to active analgesic treatment (acetaminophen/buprenorphine) or placebo for 13 weeks.
The findings, published in Clinical Interventions in Aging (16 May 2018), showed that 52.3% (n=23/44) of patients assigned to buprenorphine 5μg/hour discontinued therapy owing to adverse events, compared with 13.3% (n=6/45) of patients in the placebo group.
Neurological and psychiatric events were the most common type of adverse event in buprenorphine-treated patients.
The researchers said the findings indicated that buprenorphine was poorly tolerated in people with dementia, even at the lowest initial dose.
“When buprenorphine is administered to people with dementia, the patients’ general condition pre- and post-treatment should therefore be monitored carefully,” they concluded.
References
[1] Erdal A, Flo E, Aarsland D et al. Tolerability of buprenorphine transdermal system in nursing home patients with advanced dementia: a randomized, placebo-controlled trial (DEP.PAIN.DEM). Clin Interv Aging 2018;13:935–946. doi: 10.2147/CIA.S161052