Introduction
True allergies are rare — however, inadvertent administration of a medicine to which a patient is allergic can lead to life-threatening adverse events. Although many patients claim to have drug allergies, features of these reactions are seldom documented or properly assessed. Additionally, well known side effects of specific drugs (such as indigestion from non-steroidal anti-inflammatory drugs and abdominal upset from antibiotics) are often confused with allergies. Nevertheless, up to 50% of reported allergies are likely to be true allergic reactions;[1]
when documenting a patient’s allergy history it is essential to make clear the nature of the reaction to enable drug side effects to be distinguished from true allergic reactions.
In 2004, about 2.5 million medicines were prescribed to patients in hospitals and the community each day[2]
— the potential for medication errors and patient harm is huge. Of the medication incidents in hospitals reported to the National Patient Safety Agency in 2007, 3.2%were incidents where patients were prescribed, dispensed or administered a medicine to which they were known to be allergic, and almost a third of these incidents resulted in some harm to the patient.[3]
In order to improve patient safety and the use of medicines in the NHS, the NPSA recommended several key action points, including the need to document patients’ allergy status and to audit this aspect of clinical practice. The documentation of patients’ allergy status is an ongoing challenge in most health care systems.[4],[5]
Sandwell and West Birmingham Hospitals NHS Trust (SWBHT) comprises two separate, large and busy acute district general teaching hospitals. According to trust policies, a patient’s allergy status should be recorded on the:
- Initial assessment form
- Medical clerking (ie, the first entry in the medical notes for that admission)
- Drug chart
- Alert sheet (filed at the start of the patient’s medical notes)
- Electronic patient record (the patient admission system, which has been extended to include a record of blood results, discharge summaries, VTE risk assessments, etc)
In August 2005 the trust introduced a “red identification bracelet policy”, stipulating that patients with a history of serious adverse reactions (including anaphylactic reactions) to specific drugs must be identified with a red arm band.[6]
The aim of this audit was to assess if the documentation of patients’ allergy status was complete, and the nature of these allergies was accurate, on all of the required documents. We also sought to quantify the level of compliance with the trust’s red identification bracelet policy.
Methods
This prospective risk assessment audit was performed at SWBHT over a six-month period in 2008 and 2009. The project was discussed and logged with the trust’s clinical effectiveness department and carried out in accordance with trust policies and procedures.
The notes of 200 inpatients from two hospital sites (City and Sandwell Hospitals) were examined for recording of allergy status. To obtain a representative sample, a mixture of medical and surgical wards from both sites were audited. On each ward chosen, all patients were eligible for inclusion in the study.
For all patients, the drug chart, alert sheet, initial assessment form, medical clerking and EPR were examined to determine whether or not their allergy status was recorded. It was impossible to collect accurate data on who had completed the allergy status recording and so this was not done. The information collected was entered into an anonymous database for subsequent analysis.
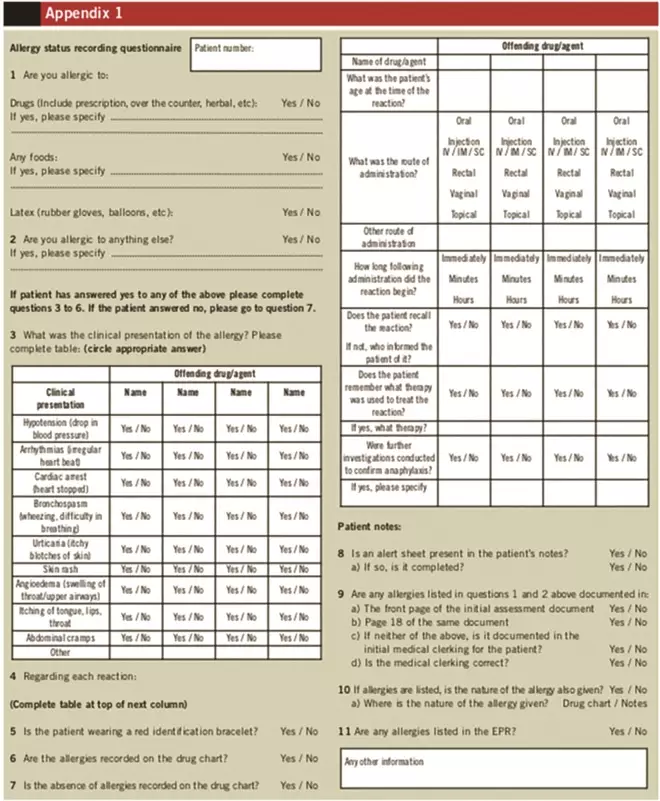
Each patient’s allergy status was verified using a standard format questionnaire, administered by one of the investigators (see Appendix 1). A pilot of the questionnaire was initially trialled on a small number of patients to ensure that it was appropriate. Patients who were asleep, too ill or disoriented, and therefore unable to take part in the questionnaire, were excluded from the audit.
Appendix 1
Patients were asked if they had any allergies to drugs or other substances such as latex or food. If the patient reported an allergy, they were asked to describe the clinical presentation of the allergy (eg, did they experience a drop in blood pressure or difficulty breathing). Two experienced clinicians reviewed the clinical details of any reported allergies to determine if they were indeed true allergies (as opposed to drug side effects). Patients were deemed to have a true allergy if they presented with any of the following groups of features:
- Hypotension, arrhythmias or cardiac arrest
- Bronchospasm, difficulty breathing or angioedema
- Muco-cutaneous manifestations such as urticaria, generalised eash, itching of tongue, lips and throat
- Abdominal cramps
If a patient reported an allergy, the presence or absence of a red identification bracelet was also recorded.
Results
A total of 200 adult patients participated in the audit, including 91 males and 109 females, with an age range of 19 to 86 years. There was an even split of patients from medical and surgical wards. The total number of patients who reported an allergy was 58. However, after analysis of their presenting clinical features only 36 of these patients were deemed to have a true allergy — the remaining 22 were believed to have experienced side effects of medicines, describing symptoms such as nausea, headache and stomach upset.
Of the patients with true allergies, the majority were allergic to penicillin (n = 22), although a large range of substances were reported, including aspirin, cefalexin, cyclizine, metoclopramide, co-codamol, barium meal and Anadin Extra (paracetamol, aspirin and caffeine). Five patients reported true allergic reactions to more than one substance.
A red identification bracelet was issued to 29 of the 36 patients with true allergies. Of the 22 patients who suffered side effects rather than true allergic reactions, 12 were wrongly issued with a red identification bracelet (see Figure 1).
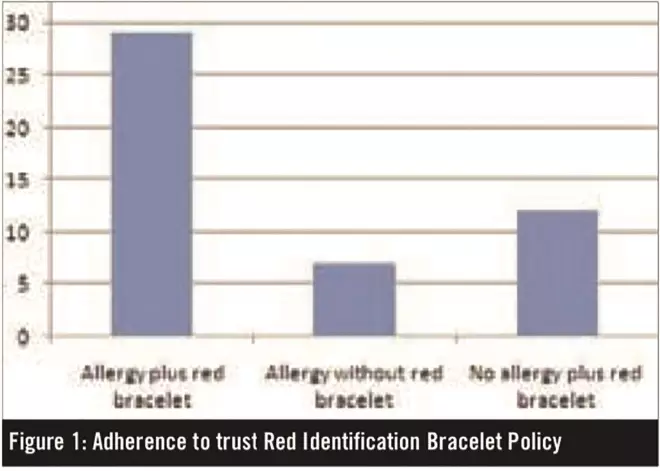
Figure 1: Adherence to trust Red Identification Bracelet Policy
Box 1 summarises the completion rate and the accuracy of allergy-status documentation. The allergy status of most patients was recorded in their drug charts and medical clerking (177 and 170 respectively; n = 200). In general, these were recorded accurately (88% of drug charts and 72% of medical clerking). The initial assessment form was sometimes used in conjunction with, or instead of, medical clerking. The alert sheet was missing in the notes of 119 patients. Allergy status was documented on one EPR, but the wrong drug was recorded.
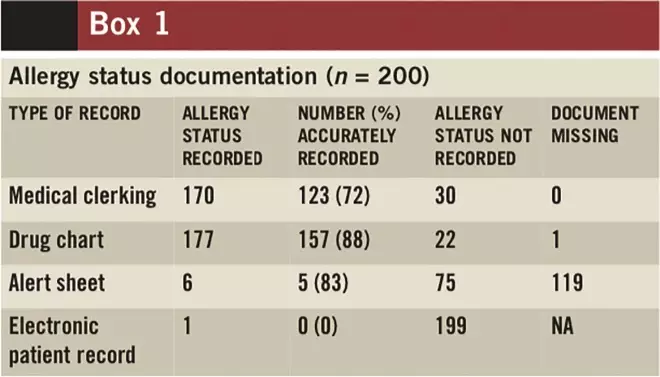
Box 1
The completion rate and the accuracy of allergy-status documentation for the 36 patients with true allergies are presented in Box 2. The patient’s allergy status was recorded in the medical clerking of only 17 of the 36 patients with true allergies. The completion rate was better in the drug charts, with 30 out of 36 patients having the documentation complete. All 30 were accurately recorded. Allergy status was documented in both the medical clerking and the drug chart for 11 patients with true allergy. Conversely, there was no record of allergy status in either the medical clerking or the drug chart for six patients with true allergies. For three patients with true allergy, their allergy status was incorrectly recorded as “no known drug allergy” in the medical clerking. Two patients were allergic to penicillin and one to both penicillin and vancomycin. The alert sheet was only used in four patients — however, all four were accurate.
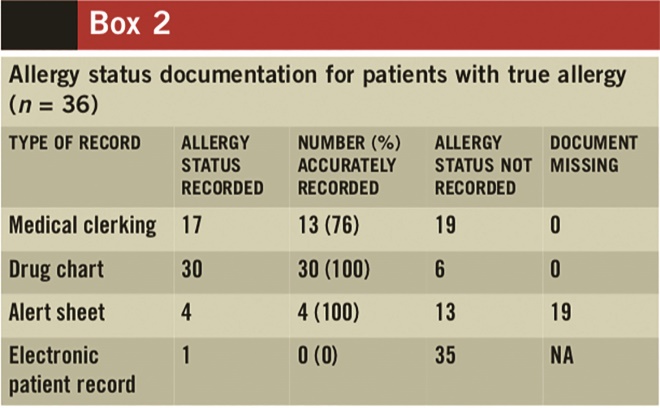
Box 2
Out of the 36 patients with true allergies, the nature of the allergy was recorded in the drug charts of only eight patients and in the medical clerking of only five patients. For one patient, this was recorded in both the drug chart and the medical clerking. The nature of allergy was not mentioned in any other type of patient documentation.
Discussion
The two main problems identified in this audit are:
- Inconsistent and inaccurate documentation
- Difficulties making a correct diagnosis of true allergy
Allergy status recording was unsatisfactory and inaccurate recording presents a serious challenge. “No known drug allergy” was incorrectly documented in three records of the 36 patients with allergies. These patients are at risk of being given a drug that might provoke an anaphylactic reaction. Conversely, 22 patients were wrongly documented as being allergic to a drug and, as a result, these patients could be denied potentially effective or life-saving medicines. In addition, there was no mention of the allergy status in either the medical clerking or the drug charts of six patients who had true allergies, which puts these patients at risk of receiving medicines to which they are allergic.
The Nursing and Midwifery Council standards for the administration of medicines require all nurses to check that patients are not allergic to medicines prior to their administration.[7]
Without proper documentation, nurses are not able to comply with these standards.
There is currently no requirement in our trust policy to record “no known drug allergy” status. However, leaving a document unrecorded gives rise to confusion about the allergy status of patients. Marking “no known drug allergy” in the allergy status section would remove any ambiguity.
It is of paramount importance that healthcare staff are made aware of the seriousness of this problem and the need for accurate recording. A regular checking system should be introduced to ensure that the documents are completed in a timely manner by appropriate staff.
The NPSA and the National Institute for Health and Clinical Excellence have produced guidance for medicines reconciliation on hospital admission.[8]
It details that, as part of the medicines reconciliation process, a detailed drug history should be obtained for all patients. This is an opportunity for a patient’s allergy status to be confirmed. It is of note in this study that more drug charts than clerking notes had a patient’s allergy status recorded, and these were also more likely to be accurate. Although data were not collected on who had completed the recording, it could be speculated that this enhanced recording was due to the activities of pharmacists and pharmacy technicians. If this was the case then implementation of medicines reconciliation gives a further opportunity to improve the recording of allergy status.
At SWBHT, neither the medicines management policy or the red identification bracelet policy makes clear who is directly responsible for documenting patient allergy status on the drug chart and in the EPR. It appears that, although the EPR is still being developed, it is not being well used (indicated by the number of incomplete records). It is essential that information on how to complete allergy-status recording in the EPR is included in staff training and that appropriate members of staff are encouraged to not only complete the records, but also to use the EPR as a source of information. If completed accurately and in a timely fashion, a patient’s EPR might even serve as a single database for allergy status recording in the future. In addition, if patients were able to access their EPR (which may happen in the future), this would help them become more involved in their own care.
Patient alert sheets were developed to highlight a number of issues at one time (for example allergy status or difficulty with intubation). This study has raised two issues with the use of the alert sheets: firstly, that less than half of the patient notes reviewed contained an alert sheet and secondly that, when they are present, they are rarely completed. In future, the EPR would be expected to take on this role. However, at the moment the EPR is not utilised to its full capability. Thought should be given to whether or not the alert sheet should be removed from the medical records or be given greater emphasis and all staff groups educated about its importance.
Many of the patients interviewed were unaware of the reason for the use of a red identification bracelet. The importance of wearing one must be conveyed especially to those with true allergies, because this will alert healthcare staff to the potential dangers.
Twenty two patients reported symptoms such as nausea, headache and stomach ache as allergy, and this was documented as such by clinical staff. This suggests that patients, healthcare staff (or both) are unaware of the differences between a drug side effect and a true allergy. Patients should be informed of the potential side effects of any medicines that are prescribed so that they are able to distinguish between true allergies and side effects. Healthcare staff should be educated to recognise the difference between the clinical presentation of true allergies and features of side effects of drugs. Accurate completion of both allergy status and nature of the allergy will also help eliminate confusion between the two.
We have developed an action plan to highlight the issues and problems identified in this study to bring about improvements in standards of patient care. This includes the following measures:
- There should be a clear policy statement on how, where, when and what needs to be recorded with regard to patient allergy status. This will require a review of trust documentation, to ensure consistency and the roles and responsibilities of clinical staff for allergy recording. There should be a positive recording policy — that is, that the absence of allergy should be positively documented as “no known drug allergy” or similar
- The trust should issue clear guidance to clinical staff on the diagnosis of true allergies. Each healthcare organisation may benefit from appointing “allergy champions” to provide leadership in promoting the safety of patients with allergies
- The above recommendations will have to be supported by a staff education and training programme. Staff must be educated about the dangers of incomplete and incorrect records
- An appropriate committee needs to be set up to action and monitor the recommendations and to determine future plans for ensuring compliance with revised and agreed policies
The results from this study have been presented to the trust clinical governance board, who have accepted the above action plan and recommendations. A trust-wide committee is being formed, led by one of the study investigators, that comprises interested nursing, medical and pharmacy staff, in addition to people from the clinical effectiveness and learning and development departments. This committee will action and monitor the recommendations and determine future plans for ensuring compliance with revised and agreed policies. We plan to repeat this audit on the completeness and accuracy of patient allergy status recording after the action plan has been implemented to measure changes.
Acknowledgements
We would like to thank our colleagues in the clinical effectiveness department for advice with regards to the design of the study and data analysis.
About the authors
Roshini Kalliat and Nicolette Smith are clinical effectiveness audit co-ordinators, Emma Graham-Clarke is consultant pharmacist for critical care and K-L Kong is a consultant anaesthetist, all at Sandwell and West Birmingham Hospitals NHS Trust.
Email: k-l.kong@swbh.nhs.uk
References
[1] Hung OR, Bands C, Laney G, et al. Drug allergies in the surgical population. Canadian Journal of Anaesthesia 1994;41:1149–55.
[2] Department of Health. Building a safer NHS for patients: improving medication safety. January 2004. www.dh.gov.uk/en/Publicationsandstatistics/Publications/Publications PolicyAndGuidance/DH_4071443 (accessed 3 September 2010).
[3] National Patient Safety Agency. Safety in doses: improving the use of medicines in the NHS 2007. www.nrls.npsa.nhs.uk/resources (accessed 3 September 2010).
[4] Basger BJ, Eckert GM. The role of the clinical pharmacist in the detection, investigation and prevention of allergic drug reactions. Australian Journal of Hospital Pharmacy 1982;12:45–8.
[5] Tempest A. Auditing the recording of allergy status in community hospitals. Hospital Pharmacist 2006;13:259–6.
[6] Sandwell and West Birmingham Hospitals NHS Trust. Red identification bracelet policy. Birmingham: 2005.
[7] Nursing and Midwifery Council. Standards for medicines management. October 2007. www.nmc-uk.org/Publications/Standards (accessed 3 September 2010).
[8] National Institute for Health and Clinical Excellence and National Patient Safety Agency. Technical patient safety solutions for medicines reconciliation on admission of adults to hospital. December 2007. www.nice.org.uk/psg001 (accessed 3 September 2010).