Abstract
Objectives
To audit the prescribing of treatment doses of low molecular weight heparins (LMWHs) with respect to clinical indication, patients’ weight and renal function. Specifically to identify the percentage of patients whose weight had been recorded on the drug chart and the number of enoxaparin doses prescribed that were 30mg or more from the calculated dose.
Methods
Preregistration trainee pharmacists in 16 Welsh hospitals collected data on the prescribing of treatment doses of LMWHs. Data were collected over a two-week period in November 2008.
Results
A total of 599 patients were identified. Renal function was reported for 80% of patients. Of the patients with renal function recorded, 87% of doses were accurate for acute coronary syndrome (ACS) and 96% were accurate for venous thromboembolism (VTE). Patient weight was recorded on 51% of drug charts. Although a weight was found for 30% of patients in other documentation, 19% of patients had no weight recorded. Both weight and renal function were documented for 68% of patients with ACS and 65% of patients with VTE. Seven patients treated with enoxaparin had a dose prescribed that was >30mg from the calculated dose.
Conclusions
Patients can be put at risk if the complete information required to dose them safely is not available. Renal function and body weight must be available within 48 hours of initiating LMWHs. We identified that patients with conditions such as atrial fibrillation, dilated cardiomyopathy, mechanical heart valves and symptomatic inherited thrombophilia are being prescribed LMWHs off-licence and that there are no consensus guidelines on dosing.
The use of low molecular weight heparins (LMWHs) has replaced the routine use of unfractionated heparin for the treatment of venous thromboembolism (VTE) and, at the time of this audit, for acute coronary syndrome (ACS).1, 2
LMWH has the advantage that the relationship between dose and response allows weight-adjusted dosing without the need for laboratory monitoring. In addition, once- or twice-daily subcutaneous dosing is possible, due to its longer half-life and increased bioavailability.3
Although there is a range of LMWH preparations available in the UK, which vary in average molecular weight (3,000 to 5,000 daltons), degree of anti Xa activity and approved indications, enoxaparin is used in most Welsh acute hospitals. However, a small number of sites in Wales use tinzaparin for the treatment of VTE.
For patients with renal impairment, adjustment of LMWH dose is required and this is based on an estimate of the patient’s glomerular filtration rate (GFR). This can be done by calculating the creatinine clearance (CrCl) from serum creatinine using the Cockcroft and Gault formula.1 Although the British National Formulary now suggests the use of an alternative formula to calculate the eGFR,1 there is currently a lack of evidence to support its use in dosing LMWHs.
The dosing schedule of enoxaparin is 1mg/kg twice daily for ACS and 1.5mg/kg once daily for VTE, for patients with normal renal function. Recently, enoxaparin has been approved for ST-segment elevation myocardial infarction (STEMI) where the dosing schedule also depends on patient age. For patients under 75 years of age the recommended dose is 1mg/kg twice a day and for those above 75 years of age the recommended dose is 0.75mg/kg twice a day.
In January 2006, the National Patient Safety Agency identified anticoagulants as high-risk medicines causing 600 cases of near harm, harm or death in England and Wales between 1990 and 2002; heparins were responsible for 23% of the 120 deaths.4 In July 2010, some 18 months after our audit, the NPSA released a rapid response report on preventing dosing errors with LMWHs. The recommendations for monitoring contained in the NPSA’s report mirror the findings in our audit. In addition, the Institute for Safe Medication Practices includes LMWH in its list of high-alert medicines.5 Anticoagulants have been identified in the 1,000 lives campaign6 and the five million lives campaign as a target for preventing harm from high-alert medicines.7
Work carried out by Allen LaPointe and colleagues8 showed that patients who received an excess dose of LMWH (>30mg from the recommended dose) had a significantly higher risk of a major bleed and in-hospital death. Unpublished work in Wales had identified enoxaparin as the second most common drug requiring a pharmacist’s intervention.
The objective of this study was to determine the proportion of patients prescribed LMWH who had their body weight recorded, renal function measured and appropriate dose prescribed (according to renal function, weight and indication).
Design and method
An investigation into the empirical prescribing of therapeutic doses of LMWHs was undertaken in 16 hospitals throughout Wales.
Preregistration trainee pharmacists in Great Britain are required to carry out an audit during their training year. There are about 50 preregistration posts across Welsh hospitals each year. In this study we used trainees to collect and submit data as part of their training. Each trainee designed and submitted their own draft data collection form to the project oversight group for review. A final data collection form was agreed by the project oversight group and was then disseminated back to each site.
Prescribing standards for this investigation were based on the dosing recommendations for enoxaparin and tinzaparin in the summaries of product characteristics and the BNF.1
Data were collected before any interventions by local pharmacy staff over a two-week period (November 2008) and entered into a central database. The location where patients’ weights were recorded was specified as: on prescription and administration record charts, on nursing documentation, in other documentation or as no weight known (and not sought). Renal function was determined using serum creatinine results from pathology and the Cockcroft and Gault formula.1 LMWH doses were calculated according to weight, renal function and clinical indication and compared with the actual prescribed doses. The proportion of doses prescribed that were >30mg from the calculated dose was recorded.
Data were collected locally and submitted centrally for collation, analysis and dissemination by the project oversight group. Local data were analysed independently by each trainee, presented at the all-Wales preregistration pharmacy training day and their hospital, if appropriate.
Ethics approval was not deemed necessary by the relevant in-house audit departments.
Results
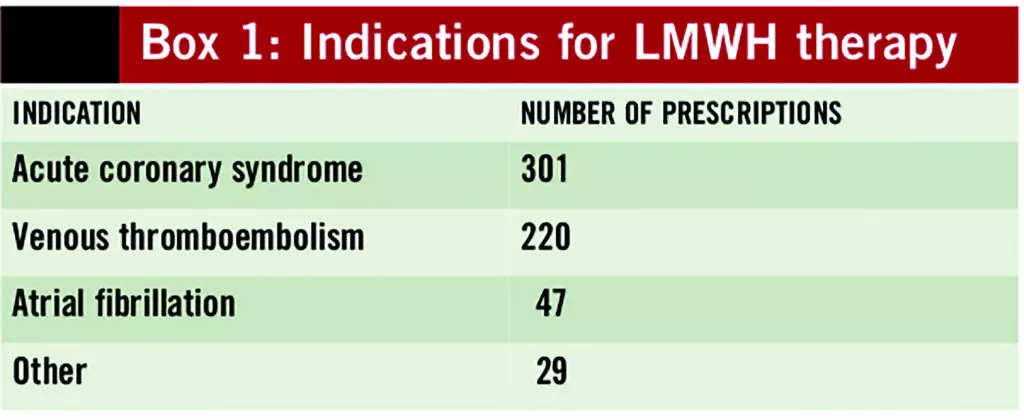
A total of 599 prescriptions for treatment doses of LMWH were identified, 94% being for enoxaparin and 6% for tinzaparin.
Indication
The most common indications for therapeutic doses of LMWH were ACS and VTE, see Box 1.
Renal function
Renal function results were available for 80% of the 599 patients reviewed.
Of the patients with their renal function recorded, the prescribing frequency for the licensed indication was appropriate in 87% and 96% of patients diagnosed with ACS and VTE, respectively.
Weight
Patient weights were recorded on 308 drug charts (51%) and in 117 nursing records (20%); they were documented in “other” places for 63 patients (10%). No weight was documented for 111 patients (19%). Of the 488 patients (50% men) with known weights, the average weights (and ranges) were 83kg for men (35–185kg) and 72kg for women (35–141kg).
Dosing
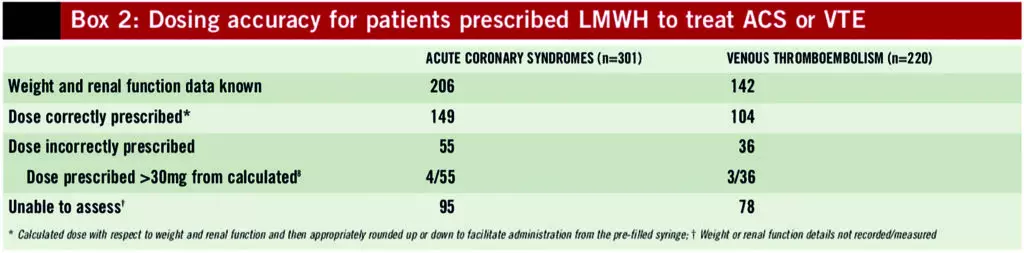
Doses and frequency of administration are based on weight and renal function. Both weight and renal function were documented for 68% of ACS patients and 65% of VTE patients.
Box 2 contains the results of dosing accuracy for the two licensed indications of enoxaparin and tinzaparin. Correct dosing can only be calculated for patients with known weight and renal function. For the patients who were prescribed incorrect doses, the number of prescribed doses greater than 30mg from the calculated dose is also reported.
Discussion
This audit examined the prescribing of therapeutic doses of LMWH for 599 patients in 16 hospitals across Wales. Evidence suggests that the prescribing of LMWH is complex, associated with prescribing errors and patient harm.4, 5, 8
Prescribing accurate doses and appropriate dosing frequency of LMWHs requires consideration of a number of factors, including: patient’s weight, renal function and diagnosis. Ideally, prescribers should know about the available dosage forms, which will support the routine practice of rounding doses up or down to an easily administered dose. This supports accurate administration by nurses.
Our results have demonstrated that only half of patients who have been prescribed therapeutic doses of LMWH have their weight recorded on the prescription chart. This figure increases to 71% when nursing documentation is included, with 19% of patients having no weight recorded anywhere. The implications of this are increased clinical risks due to over- or under-dosing of a potent drug, as demonstrated in an Australian study where increased prevalence of haemorrhagic complications were associated with failure to obtain patient weight.9
Renal function was reported 80% of the time; it is unknown what considerations were made for patients whose renal function was not recorded. However, even when poor renal function has been identified an appropriate dose modification rarely occurs. In this study the dose was appropriately modified for three out of the 41 patients for whom weight was recorded and severe renal impairment (CrCl <30ml/min) was identified.
Allen LaPointe and colleagues8 showed that higher levels of haemorrhage are likely to occur when prescribed doses are more than 30mg above or below the recommended doses, which is why we chose to consider this in our audit.
In this study 7% and 8% of ACS and VTE patients, respectively (with weight recorded on the chart and renal function known), were prescribed doses which were either greater or less than 30mg of the calculated dose. In the study carried out by Allan LaPointe, 11% of ACS subjects received a high excess dose of >30mg.
This study has identified that LMWHs are prescribed in treatment doses for unlicensed indications such as prevention of embolic events in patients with atrial fibrillation, dilated cardiomyopathy, symptomatic inherited thrombophilia or mechanical prosthetic heart valves. Prescribing of LMWHs for these conditions often occurs when patients are unable to take oral anticoagulant therapies, have had them withheld due to clinical procedures or are waiting for oral anticoagulant therapy to reach therapeutic levels. We identified that 13% of patients received LMWHs for unlicensed indications. In addition there is no consistency with respect to the doses or frequency employed for these conditions. The evidence base for these practices must be reviewed and dosing guidance standardised.
A number of training issues have been highlighted with respect to LMWH prescribing and administration, particularly relating to the availability and use of the information required for accurate prescribing such as patient weight, renal function, indication and the product presentations. Poor calculation skills may be an additional factor leading to dosing errors. The 2010 rapid response report from the NPSA states that dosing and indication variations for LMWHs have led to confusion and that readily available dosing tools can be useful to minimise these risks.10
There are several reasons why accurate patient weights may not be available, including lack of nursing time, unavailability of weighing machines at point of care and the absence of standard operating procedures indicating who is responsible for this task. Pharmacists could have a role to play here in improving documentation and availability of these essential prescribing support data.
Prescribers must also be made aware of the need to modify doses and dosing frequency in relation to renal impairment. Increasing doctors’ awareness of the available products and strengths will enable them to prescribe doses rounded to the nearest pre-filled syringe.
Some hospitals have tried to address the issues of complexity by implementing local policies to use tinzaparin for the treatment of deep vein thrombosis and pulmonary embolism whereas enoxaparin is used for patients with ACS. The numbers in this study are too small to be able to evaluate the success or otherwise of this strategy. However, this approach could be worthy of further investigation.
Co-ordinating multiple trainee projects for this type of study has benefits of increasing the sample size and power of the projects as well as utilising an “umbrella” approach to education of both trainees and supervisors by using the project oversight group for support and advice. The difficulties experienced were mainly around timely communication with, and co-ordination of, the large data collection group. Other challenges included conflict with trainee timetables in hospitals and reaching consensus on the data collection form and methodology within the project oversight group.
Lessons learnt would include the need to assign an overall project lead in the oversight group who is able and willing to drive the project forward both in terms of its design and management. This experience has demonstrated that preregistration trainee pharmacist projects can be harnessed to collect large datasets without compromising the individuals’ training. The collated work has already been presented as a poster at the British Pharmaceutical Conference.11
Conclusion
This study investigated the accuracy of the prescribing of therapeutic doses of LMWH in 599 patients. We identified that only 67% of patients prescribed LMWH for ACS or VTE (n=521) had both their weight (on prescription chart) and renal function documented, with 17% of doses being prescribed incorrectly and 8% of these being >30mg from the calculated dose.
There are training issues related to the prescribing and administration of therapeutic doses of LMWH, in particular with respect to obtaining accurate patient weights and using both weight and renal function measurements to determine the correct dose and frequency. Availability of different formulations of LMWH may complicate the picture and further work to investigate local protocols to address this would be of value. Supporting this, the NPSA has published “Reducing treatment dose errors with low molecular weight heparins”, which recommends the use of dosing tools to support safe prescribing and administration.
A number of clinical situations have been identified where unlicensed use takes place. It is essential that further work is carried out to identify the extent of this unlicensed use and also the various dosing regimens employed. Preparation of a multidisciplinary consensus statement on the appropriate dosing regimens for these conditions could then support prescribers and potentially improve clinical efficacy and safety.
This study methodology has allowed data to be collected, analysed and presented both at individual hospital and all-Wales levels thus meeting the requirements of preregistration training, local hospital and all-Wales audit methodologies. An overall project lead, however, is essential for successful collation and dissemination of data from all-Wales studies.
About the authors
By Robert McArtney, BSc, FRPharmS Sue Ashelby, DipApPharmacol, MRPharmS, Uttamlal Chouhan, MSc, MRPharmS, Janet Gilbertson, DipClinPharm, MRPharmS, James Goddard, MSc, MRPharmS, Roger Williams, BSc, MRPharmS, Mari Treharne, DipClinPharm, MRPharmS, and Sarah Hiom, PhD, MRPharmS
The authors are pharmacists at Cardiff and Vale University Health Board, Abertawe Bro Morgannwg University Health Board, Cardiff University, Betsi Cadwaladr University Health Board, Cwm Taf Health Board and Hywel Dda Health Board.
Email: sarah.hiom@wales.nhs.uk
References
- British National Formulary 60th ed. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2010.
- National Institute for Health and Clinical Excellence. Unstable Angina and NSTEMI: the early management of unstable angina and non-ST-segment-elevation myocardial infarction. March 2010. www.nice.org.uk/cg94 (accessed 5 January 2011).
- Opie LH, Gersh BJ (eds). Drugs for the Heart, 6th ed. Philadelphia: Elsevier; 2005.
- National Patient Safety Agency. Actions that can make anticoagulant therapy safer.March 2007. www.nrls.npsa.nhs.uk/resources (accessed 26 May 2010).
- Institute for Safe Medication Practices. List of high-alert medications. 2008. www.ismp.org/tools/highalertmedications.pdf (accessed 19 April 2011).
- The Health Foundation. 1,000 Lives Campaign: Wales. www.health.org.uk (accessed 26 May 2010).
- Federico F. Preventing harm from high-alert medication. Joint Commission Journal on Quality and Patient Safety. 2007;33:537–42.
- Allen LaPointe NM, Chen AY, Alexander KP, et al. Enoxaparin dosing and associated risk of in-hospital bleeding and death in patients with non-ST-segment elevation acute coronary syndromes. Archives of Internal Medicine 2007;167:1539–44.
- Hilmer SN, Rangiah C, Bajorek BV, et al. Failure to weigh patients in hospital: a medication safety risk. Internal Medicine Journal 2007;37:647–50.
- National Patient Safety Agency. Reducing treatment dose errors with low molecular weight heparins. July 2010. www.nrls.npsa.nhs.uk/resources (accessed 19 April 2011).
- McArtney R, Ashelby S, Chouhan U, et al. All-Wales audit of the prescribing of therapeutic doses of low molecular weight heparins. International Journal of Pharmacy Practice 2009;17(Suppl 2):B41.