Key points
- In the developed world, the greatest burden of disease is associated with unipolar major depression.
- Comorbidity of depression with common chronic medical conditions results in the worst health decrements of all disease states.
- The Cipriani et al. study is the most comprehensive review and analysis of the efficacy and acceptability of antidepressants to date.
- The dominant finding of the Cipriani et al. study is that when both efficacy and acceptability are considered together, three antidepressants emerge as preferred initial treatment options: agomelatine, escitalopram or vortioxetine.
- The vast majority of patients with depression stop antidepressant treatment prematurely.
- The initial choice of antidepressant is crucially important: if patients fail to respond to, or cannot tolerate the initial antidepressant, it is more difficult to achieve a response with subsequent treatments.
- There is uncertainty as to the best approach in selecting antidepressants following initial treatment failure. The most logical approach is to choose from the three preferred antidepressants identified by Cipriani et al.
- Rather than thinking of depression as a series of isolated acute episodes, management should be based on a systematic sequence of acute, continuation and maintenance phase interventions.
Introduction
In February 2018, Cipriani et al. published a systematic review of clinical trials of antidepressants for the treatment of acute episodes of major depression[1]
. Their analysis represents the most comprehensive evidence base currently available to guide the initial pharmacological treatment choice for acute major depressive disorder in adults[1]
. However, the acute phase of depression is only one aspect of the illness. For many people, depression follows a repetitive remitting and relapsing pattern, or may even develop into chronic depression. These aspects underlie the huge burden of disease associated with the illness. Although the focus of the Cipriani et al. study was focused on the acute phase, the findings must be considered within this wider context.
With increasing awareness of the crucial importance of antidepressant acceptability to patients, and the publication of the update to the National Institute for Health and Care Excellence (NICE) depression guideline postponed until December 2019, this article aims to examine the findings of the Cipriani et al. meta-analysis in the wider context of the management of depression in the UK.
Burden of depression
In 2004, the World Health Organization (WHO) published an update of its work on the global burden of disease[2]
. In the developed world, the single greatest burden of disease, defined in terms of disability-adjusted life-years, is unipolar depression — greater than the burden of ischaemic heart disease, and greater than that of cerebrovascular disease and dementia combined. Several factors, all of which contribute to disease burden, combine to make the total burden greater than the apparent sum of its parts. These include: the high prevalence of depression, which increases in the presence of other illnesses; its pervasive effect on all aspects of a person’s life; its recurrent and chronic nature; and the synergistic exacerbating effect that occurs when depression is comorbid with chronic physical conditions[2]
. This latter effect was evaluated by a World Health Survey study of nearly 250,000 participants from 60 countries[3]
. The survey used an instrument with 18 health-related questions across eight domains and compared the impact of depression with four common chronic physical diseases: angina, arthritis, asthma and diabetes. For each question, participants rated their health score from no difficulty or problem, to extreme difficulty or inability. On a 100-point scale, each of the four chronic physical illnesses was associated with an average decrease in health of between 10–12 points. The decrease associated with depression was nearly 18 points, similar to that of any two of the four physical conditions combined. The survey also found that if depression was comorbid with a physical condition, the decrement was greater still, with the greatest health deficit occurring when depression was comorbid with diabetes, resulting in a decrease of 32 points.
Using these scores, the authors concluded that depression had a greater impact on health than the common physical diseases, and that comorbidity with depression resulted in the worst health scores of all disease states[3]
.
Depression is also associated with a significant cost burden. Using data from 2012, the King’s Fund estimated the annual cost of depression to the NHS in England at around £1.8bn — approximately 21% of the total cost to the economy[4]
. Surprisingly, only a minority of NHS costs were incurred in primary care (where most cases of depression are managed) or in psychiatric inpatient care. The greatest cost burden was in the ‘other NHS’ category, and non-psychiatric inpatient care, where the interaction of depression with physical illness makes the physical illness more complex to treat and favourable outcomes more difficult to obtain[4]
. Antidepressants accounted for fewer than 5% of total NHS costs[4]
and there have been calls for depression to be managed as a chronic disease, with a systematic sequence of acute, continuation and maintenance phase interventions, rather than with the emphasis on the ad hoc management of isolated acute episodes[5],[6]
.
The Cipriani et al. study
This study evaluated antidepressant treatment in the acute phase of depression. A range of antidepressants are available to them, but uncertainty persists about which drug, or class of drugs, offers the best initial treatment option (the current clinical guideline from NICE recommends a selective serotonin reuptake inhibitor [SSRI] as initial choice)[7]
. Concerns of clinicians and researchers have centred on questions of efficacy and effectiveness[1],[8]
.
Cipriani et al.’s search strategy aimed to identify both published and unpublished double-blind, placebo-controlled and head-to-head randomised controlled trials (RCTs) of antidepressants in the acute treatment of major depression in adults (≥18 years) up to January 2016. All second-generation antidepressants approved in Europe, the United States or Japan were included (i.e. agomelatine, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, fluoxetine, fluvoxamine, levomilnacipran, milnacipran, mirtazapine, paroxetine, reboxetine, sertraline, venlafaxine, vilazodone, and vortioxetine), plus the two tricyclic antidepressants (TCAs) included in the WHO list of essential medicines (i.e. amitriptyline and clomipramine).
The scope of the search was comprehensive and included a wide range of sources: the Cochrane Central Register of RCTs; databases including PsychINFO, Embase, Medline, PSYNDEX; the websites of regulatory agencies; international clinical trial registers; and relevant pharmaceutical companies. A total of 28,522 citations were identified, but after applying inclusion and quality criteria, as well as excluding duplicate publications, this was reduced to 522 RCTs, with 116,477 participants, in studies conducted between 1979 and 2016[1]
.
Apart from the unprecedented number of studies and participants included, it is the outcome measures that make this study worthy of notice. Typically, outcomes in clinical trials of antidepressants involve changes in the number and severity of symptoms from initiation of treatment to the study end point, using instruments such as the Montgomery–Ã…sberg Depression Rating Scale (MADRS)[9]
or the Hamilton Depression Rating Scale[10]
. However, most healthcare professionals are not familiar with the clinical relevance of such changes. For this study, the primary efficacy outcome was based on whether a patient responded to treatment, defined as a minimum 50% reduction in the severity of initial symptoms at eight weeks[1]
. Similarly, ‘tolerability’ has previously been assessed by noting the frequency or severity of adverse effects and does not necessarily translate into a treatment being unacceptable to a patient. Cipriani et al. used the outcome of ‘acceptability’; simply — did the patient drop out of treatment before the end of the trial, yes or no?[1]
. Dichotomous outcomes such as these are easier for healthcare professionals to interpret[11]
. They are outcomes that can be understood intuitively and have value for patients.
Summary odds ratios (ORs) were calculated for dichotomous outcomes and standardised mean differences estimated for continuous outcomes using pairwise and network meta-analysis. Network meta-analysis is a relatively new technique that is being used increasingly in making comparisons between treatments. A simple PubMed search by the author using this term yielded more than 6,000 citations. Typically, meta-analysis involves an estimation of effect size using pairwise head-to-head comparisons. However, healthcare professionals need to be able to make judgements about treatments when head-to-head studies have never been conducted. Network meta-analysis enables a head-to-head comparison to be made even when such a study does not actually exist. Estimates of the differences between treatments are performed statistically based on logical inferences using results from several studies in which multiple treatments have been examined, with either indirect or mixed treatment comparisons involved[12]
.
Study findings — efficacy
All antidepressants in the study were found to be superior to placebo. Amitriptyline had the greatest separation from placebo (OR: 2.13; 95% credible interval [CrI]: 1.89–2.41), and reboxetine the least (OR: 1.37; 95% CrI: 1.16–1.63). The 95% CrIs of six antidepressants overlapped the limits of the 95% CrIs for amitriptyline: duloxetine, mirtazapine, nefazodone, paroxetine, venlafaxine and vortioxetine[1]
.
Head-to-head comparisons from the network meta-analysis revealed seven drugs that were superior and four drug that were inferior to the other antidepressants in the study (see Table 1).
| Superior efficacy (odds ratios from 1.19–1.96) | Inferior efficacy (odds ratios from 0.51–0.84) |
|---|---|
| Agomelatine | Fluoxetine |
| Amitriptyline | Fluvoxamine |
| Escitalopram | Reboxetine |
| Mirtazapine | Trazodone |
| Paroxetine | |
| Venlafaxine | |
| Vortioxetine |
Study findings — acceptability
Comparisons with placebo showed that agomelatine (OR: 0.84; 95% CrI: 0.72–0.97) and fluoxetine (OR: 0.88; 95% CrI: 0.80–0.96) had superior acceptability to placebo, while clomipramine was inferior (OR: 1.3; 95% CrI: 1.01–1.68)[1]
. All other antidepressants in the study were no different from placebo. Head-to-head comparisons from the network meta-analysis found that six drugs were superior and seven drugs were inferior to the other antidepressants in the study[1]
(see Table 2).
| Superior acceptability (odds ratios from 0.43-0.77) | Inferior acceptability (odds ratios from 1.30-2.32) |
|---|---|
| Agomelatine | Amitriptyline |
| Citalopram | Clomipramine |
| Escitalopram | Duloxetine |
| Fluoxetine | Fluvoxamine |
| Sertraline | Reboxitine |
| Vortioxetine | Trazodone |
| Venlafaxine |
When both efficacy and acceptability were considered together, three antidepressants emerged as superior: agomelatine, escitalopram and vortioxetine. Another three antidepressants emerged as inferior: fluvoxamine, reboxetine and trazodone[1]
. In terms of informing clinical decisions, the three superior antidepressants might be considered first-choice treatments unless there are compelling reasons not to use them, while the three inferior agents should be avoided.
Context, uncertainty and patient acceptability
The findings of Cipriani et al. are comprehensive and, if taken at face value, compelling. But what influence should they have on practice? The findings must be viewed in the context of the different phases of depression and the extraordinarily high burden of illness, as well as current practice and guidelines.
The current NICE guideline for the management of depression was first published in 2009 and was updated in 2016 and 2018[7]
, with a further update due at the end of 2019. The guideline stipulates that in moderate-to-severe acute depression, the initial choice of antidepressant should normally be “an SSRI in generic form because SSRIs are equally effective as other antidepressants and have a favourable risk–benefit ratio”[7]
. Should this change in the light of the findings of Cipriani et al.? The statement that SSRIs are equally as effective as other antidepressants and have a favourable risk–benefit ratio can no longer be taken at face value. However, an SSRI in generic form can still be the presumptive first choice providing it is one of the three superior antidepressants identified by Cipriani et al. (in this case, escitalopram). There is, however, uncertainty about what to do next if the patient fails to respond or stops treatment prematurely.
The biggest problem encountered in treating depression with an antidepressant is acceptability[13]
. The key to successful antidepressant treatment is keeping the patient on it for long enough. The NICE guideline states that antidepressants should be continued for a minimum of six months following remission of a major depressive episode, possibly longer, depending on the presence of residual symptoms and previous episodes of depression. For patients at risk of relapse, antidepressant treatment should continue for at least two years[7]
. Unfortunately, the vast majority of patients stop treatment prematurely[13]
. The extent of this problem was suspected as early as the 1980s but was confirmed in a series of studies in the UK in the 1990s and 2000s[14],[15],[16]
.
In 1999, a large population-based study involving more than 16,000 patients compared the treatment patterns of SSRIs and TCAs in patients with a diagnosis of depression from a GP[14]
. Based on the UK Defeat Depression Campaign recommendation[17]
that ‘adequate’ treatment consisted of taking an effective antidepressant dose for four consecutive months, the OR for completing adequate treatment on an SSRI compared with a TCA was 7.5 (P <0.001). Overall, only 6% of patients prescribed a TCA received adequate treatment. However, SSRIs are not perfect; a similarly large study of patients prescribed fluoxetine, paroxetine or sertraline found that nearly half of all patients discontinued treatment during the first month and that, overall, only 30.8% entered a fourth month of continuous treatment (fluoxetine: 31.1%; paroxetine: 29.9%; sertraline: 23.9%)[15]
. The differences in attrition rates between fluoxetine and sertraline, and paroxetine and sertraline were statistically significant (fluoxetine vs. sertraline Χ
2 =25.61, P <0.001; paroxetine vs. sertraline Χ
2 =11.28, P <0.001). More recently, a very large study using the General Practice Research Database, involving patients diagnosed as depressed by their GP and prescribed an antidepressant, found that in the final year of the study (2005), 78% of nearly 200,000 patients took an antidepressant for fewer than 30 days, and only 1.8% took an antidepressant for longer than 60 days[16]
.
Clearly, from the moment an antidepressant is prescribed, healthcare professionals involved in or advising on prescribing should prepare for the almost inevitable likelihood that the patient will stop treatment prematurely. This may be because the treatment has not worked, because of adverse effects, or it may be for other reasons. Choosing a ‘better’ initial antidepressant — one of either agomelatine, escitalopram and vortioxetine, identified by Cipriani et al.[1]
— may help keep patients in treatment for longer. But what should be done if the initial treatment is ineffective or unacceptable?
The NICE guideline recommends that in cases of inadequate response or intolerable adverse effects, the antidepressant should be switched: “initially to a different SSRI or a better-tolerated newer antidepressant” and “subsequently an antidepressant of a different pharmacological class that may be less well tolerated, for example venlafaxine, a TCA or a monoamineoxidase inhibitor (MAOI)”[7]
. Before considering these recommendations in the light of Cipriani et al.’s findings, there is more evidence to be explored.
Perhaps the best designed and most robust study to investigate antidepressant sequencing following initial treatment failure is the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study[18]
. Patients with moderate-to-severe major depression all started treatment with citalopram (step one). Patients who did not respond adequately or who could not tolerate citalopram were offered the choice of several treatments: buproprion (which is not licensed as an antidepressant in the UK), sertraline, venlafaxine, citalopram plus buproprion, or citalopram plus buspirone (step two). The option of psychotherapy was also available, but this aspect of the study will not be discussed here. Patients with an inadequate response or who could not tolerate treatments at step two were then offered the choice of more treatments: nortriptyline, mirtazapine, augmentation with lithium or with triiodothyronine (step three). In the final stage (step four), if patients did not respond to, or could not tolerate, step three treatments, they were then offered the choice of either tranylcypromine or venlafaxine combined with mirtazapine. As patients moved up from one step to the next, the study anticipated that obtaining a treatment response was likely to be more difficult and the intensity of treatment was escalated accordingly. Results for steps one to three showed that the proportions of patients responding to treatment decreased from 48.6% in step one to 16.8% in step three, with no further reduction in step four. Similarly, the proportions of patients achieving remission fell from 36.8% in step one, to 13.7% in step three and 13.0% in step four. By contrast, the proportions of patients experiencing intolerable side effects increased from 16.3% in step 1 to 30.1% in step 4 (see Figure). Increasing the intensity of treatment did not improve response, but it did increase the incidence of unacceptable side effects. These findings indicate the crucial importance of the initial choice of antidepressant at step one and step two.
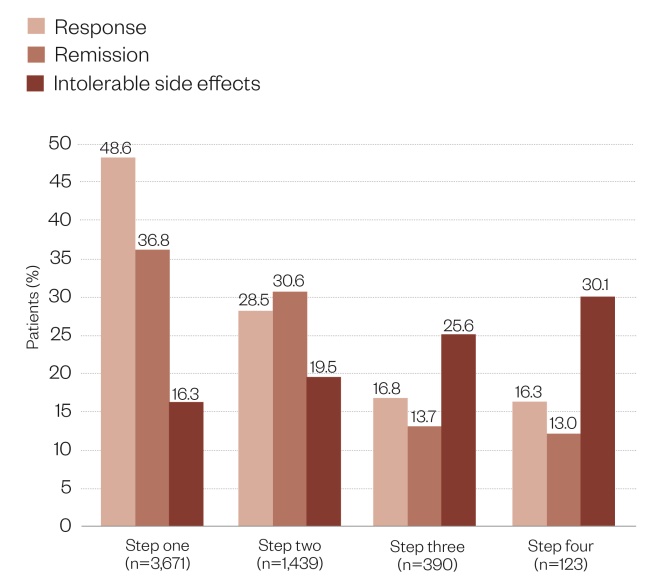
Figure: Outcomes of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study
Source: Rush JA, Trivedi MH, Wisniewski SR et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905–1917
Cipriani et al. identified three antidepressants as the best first-choice options: agomelatine, escitalopram or vortioxetine. A NICE technology appraisal guideline (TAG) was published for one of these, vortioxetine, in November 2015[19]
. Vortioxetine is recommended as a third-line treatment option for patients with major depression who have not responded adequately to two antidepressants within the current episode — a very different recommendation from Cipriani et al., with a similar conclusion reached by the Scottish Medicines Consortium (SMC)[20]
. Both sets of guidance are based on economic models submitted by the manufacturers (Lundbeck Ltd) that estimated the cost-effectiveness of vortioxetine when used as a third-line treatment over a time scale of two years. On reading both sets of guidance, it becomes clear that there is no clinical evidence to inform the use of vortioxetine in this way. The NICE TAG acknowledges this, but states that “evidence from trials in the first-line population was relevant to informing the relative effectiveness of vortioxetine compared with other antidepressants for second and subsequent lines of treatment”[19]
. What this assertion is based on is not mentioned.
The STAR*D study showed that the outcomes obtained from third-line treatments were markedly different from those obtained at first line[18]
. The cost-effectiveness estimates and recommendations for third-line use were derived solely from health-economic models that were based on multiple unproven assumptions. For example, rates of remission and lack of treatment response after two treatment attempts (i.e. at the point where third-line treatment would be initiated) were obtained by using data from the STAR*D study relating to the reduction in efficacy from step two to step three, ignoring the fact that STAR*D was conducted in the United States and the treatment pathways were very different from both recommendations and practice in the UK. However, both the NICE TAG and the SMC guidance accepted that, in the short term at least, patients treated with vortioxetine were likely to experience fewer adverse effects and were more likely to continue antidepressant treatment than would be the case with most other antidepressants, making the restriction of this drug to third-line use illogical[19],[20]
.
Returning to the question of how to proceed if the initial treatment is ineffective or unacceptable, it should be noted that there is a paucity of evidence and that the study by Cipriani et al. is concerned with initial treatment only. A 12-week RCT of vortioxetine versus agomelatine in patients who had experienced an inadequate initial response to an SSRI or a serotonin-noradrenaline reuptake inhibitor found that symptom ratings (change in mean MADRS total score from baseline to endpoint) improved by over 50% for both drugs[21]
. By week 12, 70% of vortioxetine-treated patients had responded to treatment and 55% were in remission, and vortioxetine was statistically and clinically superior to agomelatine (number needed to treat [NNT] for response to treatment with vortioxetine compared with agomelatine = 7; 95% CI: 4–17; NNT for remission = 6; 95% CI: 4–14. NNTs calculated by the author). The results must be treated with some caution: the inadequate response to initial treatment was based on the mean MADRS total score at study entry without noting the severity of depression when the initial treatment had been started, or what the response to that treatment had actually been. Moreover, potential reasons for the lack of response, which could have included poor adherence to the initial treatment (which does not equate to inadequate response), were not reported[21]
. However, despite the lack of precision in defining the patient population or their history, it is likely that these patients would be similar to those seen by GPs in the UK: a diverse population, some of whom had tried the initial treatment and failed to respond, and some of whom had found it unacceptable. When viewed in this way, the results assume a different significance.
This still does not answer the question of how to proceed if the initial treatment is ineffective or unacceptable. The NICE depression guideline states that “evidence from primary studies of existing treatments should also be considered when making decisions about second and subsequent lines of treatment”[7]
. In the absence of well-designed and well-conducted trials of antidepressants as second-line or third-line treatments, this is the only option. As a second-line treatment, the recommendation is for a switch to a different SSRI or a better tolerated newer antidepressant and, at third-line, an antidepressant that may be less well tolerated, such as venlafaxine, a TCA or an MAOI[7]
. NICE states that the evidence for this is weak. The NICE TAG and SMC guidance for vortioxetine position this drug for third-line use, even though it is not less well tolerated[19],[20]
. The findings of Cipriani et al. create a new perspective from which these recommendations should be viewed.
Conclusion
Bringing together the different threads of evidence, since all licensed antidepressants have proven efficacy, it is clear that acceptability must be the most important consideration when selecting a treatment. The evidence that patients stop antidepressant treatment prematurely is overwhelming[13],[14],[15],[16]
. The STAR*D study found that by the time step three was reached, a good outcome was much more difficult to achieve[18]
. Choosing an acceptable antidepressant at the earliest opportunity is crucially important. The findings of Cipriani et al. are clear: the three superior antidepressants, agomelatine, escitalopram and vortioxetine, should all be considered as first-line treatment options. If escitalopram is used first, it makes no sense to switch to second-line treatment with another SSRI that is inferior in either efficacy or acceptability to the first. As alternatives to escitalopram, the study by Cipriani et al. identified two better-tolerated newer antidepressants: agomelatine and vortioxetine. These should be considered as second-line treatment options (or first-line if escitalopram is unsuitable). Given the enormous burden of depression — clinical, societal and financial — to try to minimise treatment costs by insisting on the use of antidepressants that have a lower acquisition cost but also an inferior profile of efficacy and acceptability is surely a false economy and is not in the best interests of patients.
Financial and conflicts of interest disclosure
The author has received honoraria for speaking at educational meetings or fees for consultancy services from the following organisations: DB Ashbourne Ltd., The College of Mental Health Pharmacy, Lundbeck Ltd., Open Health Communications Ltd., Otsuka UK Ltd., Sunovion Ltd., Sussex Partnership NHS Trust and Virgo Health Ltd. No fee was sought or offered from any party in respect of this article. No writing assistance was used in the production of this manuscript.
References
[1] Cipriani A, Furukawa TA, Salanti G et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7
[2] World Health Organization. The Global Burden of Disease: 2004 update. 2004. Available at: https://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/ (accessed April 2019)
[3] Moussavi S, Chatterji S, Verdes E et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007;370:851–885. doi: 10.1016/S0140-6736(07)61415-9
[4] McCrone P, Dhanasiri S, Patel A et al. Paying the price: the cost of mental health care in England to 2026. King’s Fund. 2008. Available at: https://www.kingsfund.org.uk/publications/paying-price (accessed April 2019)
[5] Andrews G. Should depression be managed as a chronic disease? BMJ 2001;322:419–421. doi: 10.1136/bmj.322.7283.419
[6] Scott J. Depression should be managed like a chronic disease. BMJ 2006;332(7548):985–986. doi: 10.1136/bmj.332.7548.985
[7] National Institute for Health and Care Excellence. Depression in adults: recognition and management. NICE guideline [CG90]. 2018. Available at: https://www.nice.org.uk/guidance/cg90 (accessed April 2019)
[8] Donoghue JM & Hylan T. Antidepressant use in clinical practice: efficacy vs. effectiveness. Br J Psychiatr 2001;179(suppl 42):s9–s17. doi: 10.1192/bjp.179.42.s9
[9] Montgomery SA & Ã…sberg M. A new depression scale designed to be sensitive to change. Br J Psychiatr 1979;134(4):382–389. doi: 10.1192/bjp.134.4.382
[10] Hamilton, M. Rating depressive patients. J Clin Psychiatry 1980;41(12):21–24.
[11] Cipriani A, Furukawa TA, Salanti G et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5
[12] Shim S, Yoon BH, Shin IS & Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol Health 2017;39:e2017047. doi: 10.4178/epih.e2017047
[13] Donoghue JM & Taylor DM. Sub-optimal use of antidepressants in the treatment of depression. CNS Drugs 2000;13a(5):365–383. doi: 10.2165/00023210-200013050-00006
[14] Dunn RL, Donoghue JM, Ozminkowski RJ et al. Longitudinal patterns of antidepressant prescribing in primary care in the United Kingdom: comparison with treatment guidelines. J Psychopharmacol 1999;13(2):136–143. doi: 10.1177/026988119901300204
[15] Donoghue JM. Selective serotonin reuptake inhibitor use in primary care: a five-year naturalistic study. Clin Drug Invest 1998;16(6):453–462. doi: 10.2165/00044011-199816060-00005
[16] Moore M, Yuen HM, Dunn N et al. Explaining the rise in antidepressant prescribing: a descriptive study using the general practice research database. BMJ 2009;339:b3999. doi: 10.1136/bmj.b3999
[17] Paykel ES & Priest RG. Recognition and management of depression in general practice: consensus statement. BMJ 1992;205:1198–1202. doi: 10.1136/bmj.305.6863.1198
[18] Rush JA, Trivedi MH, Wisniewski SR et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905
[19] National Institute for Health and Care Excellence. Vortioxetine for treating major depressive episodes: Technology appraisal guidance [TA367]. 2015. Available at: https://www.nice.org.uk/guidance/ta367 (accessed April 2019)
[20] Scottish Medicines Consortium. Vortioxetine 5mg, 10mg, 20mg film-coated tablet (Brintellix) SMC No. (1158/16) 2016. Available at: https://www.scottishmedicines.org.uk/medicines-advice/vortioxetine-brintellix-fullsubmission-115816/ (accessed April 2019)
[21] Montgomery SA, Nielsen RZ, Poulsen LH & Haggstrom L. A randomised, double-blind study in adults with major depressive disorder with an inadequate response to a single course of selective serotonin reuptake inhibitor or serotonin-noradrenaline reuptake inhibitor treatment switched to vortioxetine or agomelatine. Hum Psychopharmacol Clin Exp 2014;29(5):470–482. doi: 10.1002/hup.2424


