Key points
- Increased drug clearances during pregnancy may lower circulating antiepileptic drug concentrations, resulting in loss of seizure control.
- Intrauterine exposure to certain antiepileptic drugs, particularly valproate, may be associated with the development of foetal malformations.
- Intrauterine valproate exposure is also associated with lower IQ values, neurodevelopment delay and autism.
- As far as possible, prescription of valproate in women capable of pregnancy is better avoided.
Introduction
The safety of antiepileptic drug use in pregnancy involves: the pregnant woman in her own right; the foetus while in her womb; and during its subsequent extra-uterine existence as a neonate and infant. The safety of antiepileptic drugs during pregnancy is not significantly different from the safety of these drugs in women in general, except in relation to consequences of pregnancy affecting the female body’s handling of the drugs, and the drugs’ effects on the foetus in utero and afterwards. This article focuses on these additional pregnancy-related safety aspects, and not with more extensive matters of antiepileptic drug safety in general.
In recent years, antiepileptic drugs have been increasingly used in treating disorders other than epilepsy. So much so that Bobo et al. reported that, in the United States, these drugs were more often prescribed for psychiatric disorders than for their original indication of epileptic seizure control[1]
. In that study, antiepileptic drugs had been taken in 2.2% of more than 500,000 pregnancies, in 47.9% being indicated for various psychiatric disorders, 22.0% for pain management and 20.7% for epilepsy. Therefore, antiepileptic drugs were taken for epilepsy by 0.4–0.45% of pregnant women, consistent with the 0.5% prevalence figure for active epilepsy that seems to apply in Western societies. Despite this considerable use for disorders other than epilepsy, nearly all the available scientific information relating to the clinical pharmacology of these drugs in pregnancy has been derived from studies on their use in women with epilepsy, and their employment in other disorders has depended on application of this experience. Consequently, this article is mainly concerned with the use of these drugs in pregnant women who have epileptic seizure disorders.
Sources
This article is based on a personal collection of the relevant English language literature, dating from the 1970s, and on publications traced through the PubMed database, searched using the term antiepileptic drug, in combination with, individually, the terms: epilepsy, foetus, malformation, neonate, neurodevelopment, pregnancy, seizure, and teratogenesis. Some material drawn from the Australian Register of Antiepileptic Drugs in Pregnancy is also included. Since this register includes pregnancies in women with epilepsy who did not take antiepileptic drugs in at least the earlier half of pregnancy, its data can be used to help discriminate between the effects of antiepileptic drug exposure itself and the combined effects of the drugs plus having epilepsy.
Discussion
The pregnant woman: seizure control
There appears to be little or no evidence that antiepileptic drug use in pregnancy produces additional safety concerns for the woman involved, beyond those that result from decreased control of her epileptic seizures. Reasonably effective antiepileptic drug treatment has been available since 1857, and there has been little published on untreated epilepsy in pregnancy for many years. Half a century ago, the predominant view was that epileptic seizure control tended to deteriorate during pregnancy. However, the published studies, included in the review of Schmidt[2]
, were not consistent in showing this. Overall, more patients had fewer seizures during pregnancy, but more of the studies, including the often cited and reasonably sized investigation of Knight and Rhind[3]
, found tendencies to worsening seizure control, as did Schmidt et al’s own study[4]
. The interpretation of these relatively early studies and much of the subsequent data have often been confounded by issues relating to antiepileptic drug use and prescribed drug dosages.
Worsening control of epileptic seizures could expose the pregnant woman to risk of physical injury and possibly death during seizures, and there are reports of such events[5]
. However, in 2004 an increased mortality rate was found in pregnant women with epilepsy in the UK, mostly attributable to sudden unexplained death[6]
. In addition to physical injury, loss of seizure control could lead to psychological harm to the woman with epilepsy, which may result in reduced social activities and opportunities and may impair her quality of life, particularly if seizures cause the loss of her driving licence, for example.
Since the mid-1970s, a number of studies have shown that, as pregnancy progresses, steady state plasma concentrations of antiepileptic drugs tend to fall unless the drug doses are increased[7],[8]
. The factors involved in producing this fall appear to be: increased renal clearances of unmetabolised drug because of the physiological increase in glomerular filtration rate that begins early in pregnancy, a phenomenon that is of greatest importance for antiepileptic drugs that undergo relatively little (e.g. levetiracetam) or no metabolism (e.g gabapentin) in the body; increased biotransformation, because increased circulating levels of female steroidal sex hormones during pregnancy induce formation of the cytochrome P (CYP) 450 isoenzymes and glucuronosyl transferases that play the main roles in eliminating most antiepileptic drugs; and a dilutional effect from the increased maternal extracellular fluid, womb, placental and foetal volumes during pregnancy, an effect offset to an extent in late pregnancy when plasma albumin concentrations fall, so that there is an increased concentration of unbound (biologically active) drug relative to total drug in plasma. The extent of the decreases in plasma antiepileptic drug concentration caused by these factors varies between the individual drugs and the stage of pregnancy. The decreases tend to be relatively small for agents that are cleared from the body predominantly by renal excretion unmetabolised, and are larger for drugs cleared mainly through biotransformation. The greatest clearance increase among the currently commonly used antiepileptic drugs is that for lamotrigine, eliminated largely by N-glucuronidation. Doses of this drug may need to be doubled or tripled during pregnancy to maintain steady-state plasma drug concentrations at their pre-pregnancy values. In contrast, the fall in plasma carbamazepine concentration tends to be relatively small and not statistically significant when the drug is used as the sole antiepileptic agent in pregnancy, but is appreciably greater when it is employed in combination with phenytoin or phenobarbitone[9]
. The fall in plasma concentrations of valproate during pregnancy is also usually relatively small. CYP450 isoenzymes play a relatively small role in valproate elimination, which mainly depends on fatty acid β-oxidation and on acyl glucuronidation of the parent molecule.
It is generally accepted that seizure control tends to correlate with plasma antiepileptic drug correlations. This raises the possibility that, unless antiepileptic drug doses are adjusted, falling circulating concentrations of antiepileptic drugs in pregnancy may explain any deterioration in seizure control that occurs. The clinical impression has developed that, if antiepileptic drug doses are adjusted during and after pregnancy, so that plasma drug concentrations are maintained at their pre-pregnancy values in the individual, seizure control is unlikely to deteriorate. This impression has been supported by recent studies in which a dosage adjustment policy, particularly when applied to lamotrigine, seems to have avoided worsening seizure control in pregnancy[10],[11],[12]
.
This avoidance of loss of seizure control associated with appropriate adjustment of antiepileptic drug dosage during pregnancy might suggest that the state of pregnancy itself does not enhance seizure genesis. However, a recent investigation has produced some evidence that seizures increase during pregnancy in women with epilepsy who are not being treated with antiepileptic drugs[13]
.
For practical purposes, the safety of antiepileptic drug therapy for pregnant women will probably not be endangered by consequences of loss of seizure control if there is careful management of antiepileptic drug dosages throughout, and after, pregnancy, guided when feasible by plasma antiepileptic drug concentration monitoring.
Course of pregnancy
The available literature does not permit any easy distinction being made between the possible effects of loss of control of epilepsy, and those caused by its drug treatment, during the course of pregnancy. There seem to be no unequivocally demonstrated effects.
The violence of maternal convulsive seizures might be expected to increase the risk of intrauterine foetal damage, possibly causing miscarriage, placental abruption, retro-placental haemorrhage or premature birth. However, there have been surprisingly few reports of such complications occurring that are associated with convulsive seizures during pregnancy. In 1964, Janz and Fuchs[14]
found more miscarriages and stillbirths had occurred in antiepileptic drug-treated pregnancies than in the pregnancies of women with untreated epilepsy, though post-mature and premature births were not more frequent. In 1972, Speidel and Meadow[15]
failed to find any increase in rates of miscarriage, pre-term birth and low birthweights in antiepileptic drug pregnancies and Annegers et al
[16]
arrived at a similar conclusion in relation to spontaneous abortions. Stillbirth rates were not increased in the pregnancies of adequately treated women with epilepsy[17],[18]
. McPherson et al
[19]
also found no increase in stillbirths, or in premature births in women with epilepsy. Interestingly, in a large contemporaneous database there was no increase in spontaneous abortion rates in almost 1 million Danish pregnancies that occurred in women with antiepileptic drug-treated epilepsy[20],[21]
. However, in the same data set, there was an increased spontaneous abortion rate in pregnant women who took these drugs for disorders other than epilepsy, a finding that is difficult to explain.
Katz et al
[18]
noted a statistically significant increased rate of caesarean sections in the pregnancies of women with epilepsy; at least the majority of whom were taking antiepileptic drugs. Borthen and Gilhus[22]
described a similar finding and also noted an increased incidence of pre-eclampsia, gestational hypertension and induced labour. Other, sometimes larger scale, studies have shown inconsistencies between such findings in relation to antiepileptic drug-treated pregnancies. It is possible that the differences in reported outcomes regarding some of these matters reflect standard management practices in different institutions more than courses of action made desirable by actual events in individual pregnancies.
Overall, it seems reasonable to conclude that, in the considerable majority of instances, antiepileptic drug use in pregnancy probably does not adversely affect, or unduly complicate or endanger, the course of pregnancy, despite occasional reports to the contrary such as that of Rauenszaucher et al
[23]
.
The foetus, in utero: foetal growth and health
The majority of reports have shown that there is a tendency for babies exposed to antiepileptic drugs in utero to be a little premature, small for gestation age and of lower than expected birth weight [24],[25]
. Their head circumferences also tends to be smaller, but by the age of two years the circumference values fall within the normal range for the population. Such outcomes of pregnancy are unlikely to have any significant overall deleterious consequence for foetal safety and subsequent well-being. The respective contributions from having epilepsy, and from taking drug treatment for epilepsy, to the origins of these abnormalities have not been clarified. However, Finnish population data exist that show increased neonatal death rates in association with previous in utero antiepileptic drug exposure[26]
, with a further study reaching a similar conclusion[27]
. The explanation for this increased death rate, and its relation to antiepileptic drug exposure in utero rather than to the mother suffering from epilepsy, are both unclear.
Foetal structural malformation
The issue of foetal malformation associated with antiepileptic drug exposure during pregnancy began to arise soon after the thalidomide tragedy of the 1950s. The influential early study of Janz and Fuchs[14]
in 1964 concluded that such drug exposure was unlikely to be responsible for foetal malformations. This was despite there being a 1.4% incidence of malformation in these authors’ 348 antiepileptic drug exposed pregnancies, but no instances in the offspring of a further 130 pregnancies in women with epilepsy from their series who did not take antiepileptic drugs during pregnancy. After 1970, a series of publications began to appear, first comprising small case series before later including larger data collections. The majority provided evidence, though it was not always statistically significant, that there was an increased hazard of foetal malformation in infants born to mothers who had taken antiepileptic drugs during pregnancy (originally mainly phenytoin, phenobarbitone, or a drug biotransformed to the latter). The view developed that, as a class, antiepileptic drugs were teratogens. However, unlike the situation in relation to thalidomide, which appears to have been responsible for a particular pattern of malformation, foetal exposure to antiepileptic drugs seemed to be associated with a considerable variety of structural maldevelopments in exposed foetuses. The abnormalities ranged from comparatively trivial deformities of the skin or digits through significant, though potentially surgically remediable, malformations, such as cardiovascular abnormalities and facial clefts, to devastating and uncorrectable ones (e.g. spina bifida, meningomyelocoele), some being incompatible with continuing extrauterine existence (e.g. anencephaly). ‘Foetal anticonvulsant’, ‘foetal phenytoin’, and ‘foetal valproate’[28]
syndromes began to be described, though there was considerable overlap between the various abnormalities of development included in each. It seems rather doubtful whether these syndromes represent genuine discrete drug-specific entities.
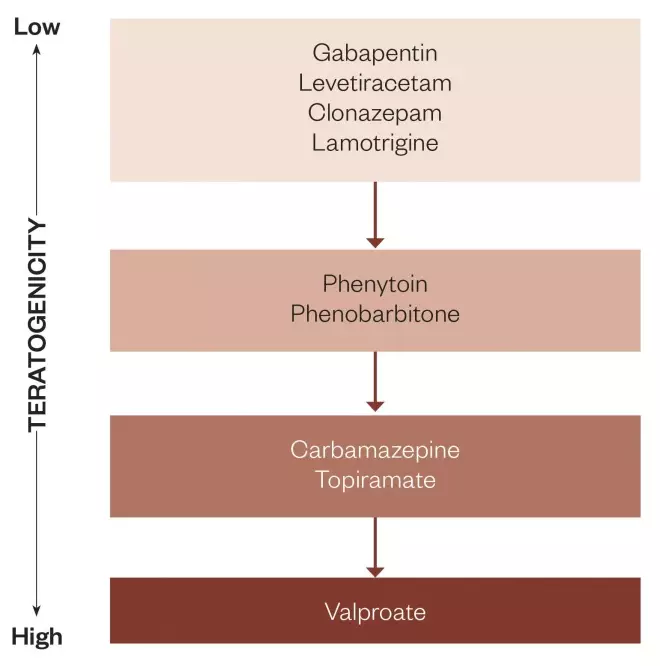
Figure 1: Degrees of teratogenicity for antiepileptic drugs: Based on Australian Pregnancy Register data and other literature, gradient of increasing teratogenicity for antiepileptic drugs for which sufficient statistical information is available. The top group appears safe, for practical purposes, though the data for gabapentin are relatively scanty. The second group has been suspect, particularly phenobarbitone. The third group appears to be dose-related teratogens, though not particularly potent ones, whereas valproate is a significant teratogen.
With the subsequent publication of larger data sets, including material collected in various antiepileptic drug pregnancy registers (mainly in the UK, North America, Australia and Europe [EURAP]) and in governmental and institutional databases, the situation regarding antiepileptic drug-associated foetal malformation has become clearer. Nevertheless, significant deficiencies in knowledge remain. Without working systematically through each development in this area, one important point that has emerged is that foetal malformation is not a class effect that involves all antiepileptic drugs. There is now reasonably persuasive evidence that the degree of teratogenicity varies between different antiepileptic drugs (see figure 1). It seems beyond reasonable doubt that valproate is a definite teratogen, and that the risk of foetal malformation associated with exposure to it increases with increasing valproate dose taken in the earlier part of pregnancy[29],[30],[31]
(see figure 2). More recent data have shown that there is a degree of dosage-related specificity in the pattern of foetal abnormality associated with valproate exposure. Thus the risk of spina bifida appears low until maternal valproate dosage exceeds 1650mg/day[32]
or 2000mg/day[33]
, whereas other types of malformation may be associated with lower dosages. A meta-analysis of the valproate-associated foetal malformation literature is available[34]
.
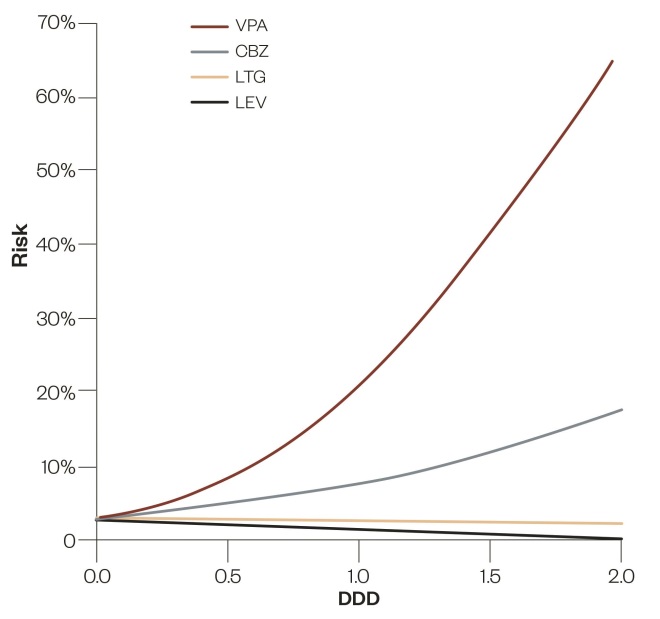
Figure 2: Logistic regressions for foetal malformation risk on epileptic drug dose: Logistic regressions for foetal malformation risk on drug dose for valproate (VPA), carbamazepine (CBZ), lamotrigine (LTG) and levetiracetam (LEV) in monotherapy, based on the most recent version of Australian Pregnancy Register data. To bring them to a common basis, drug doses are expressed as multiples, or sub-multiples, of the WHO Defined Daily Doses (DDDs) of these drugs, 1500mg, 1000mg, 300mg and 1500mg per day, respectively.
Whereas valproate-associated teratogenicity seems to be a reasonably securely demonstrated phenomenon, there are several other commonly used antiepileptic drugs for which there is some evidence of human teratogenicity, though not all studies have shown associations that are statistically significant. Such drugs include topiramate, carbamazepine, phenobarbitone and phenytoin. In the case of topiramate, some studies have shown probable and sometimes statistically significant associations with foetal malformation in general[35]
or with particular malformations, especially oral clefts and microcephaly[36]
. Data from the Australian Pregnancy Register show no association at all when the drug has been taken in pregnancy as the sole antiepileptic agent (in 42 pregnancies), but a statistically significant and dose-related one with foetal malformations in general when the drug was employed with another antiepileptic drug (in 85 pregnancies), though no particular culpable drug combination could be identified[37]
. While some earlier studies tended to exculpate carbamazepine as a teratogen, subsequent and larger scale investigations have tended to incriminate it[38],[39]
. Australian Pregnancy Register data provide statistically significant evidence that carbamazepine, if used as the sole antiepileptic agent, is associated with a roughly doubled risk of foetal malformation compared with reasonably well-matched comparator pregnancies[40]
. North American studies[41]
and the EURAP Pregnancy Registry data[42]
, which may have included some of the North American material, provided evidence for the teratogenicity of phenobarbitone, though earlier investigations of this possibility had failed to do so. In contrast, the earlier evidence suggesting that phenytoin use was associated with foetal malformations has not been supported at a statistically significant level by the accumulation of more extensive data[43]
. Enough material now appears to be available to suggest that lamotrigine is comparatively safe from foetal malformation. Earlier, the UK Register data appeared to show foetal malformation rates rising with increasing lamotrigine dosage[44]
, but this finding was not sustained at a statistically significant level in the investigation of an expanded data set drawn from the same register[45]
. Other studies based on medium-sized databases have failed to demonstrate statistically significant increased malformation rates associated with lamotrigine, though the rates have often tended to be higher than those in comparator populations without epilepsy. Several separate studies[37],[46],[47]
and a meta-analysis[48]
have now shown such low foetal malformation rates associated with levetiracetam monotherapy in pregnancy that the drug seems unlikely to possess any appreciable degree of teratogenicity. A similar conclusion may apply for gabapentin[49]
, though the available evidence base is relatively small, and for benzodiazepine antiepileptic agents, clonazepam in particular.
Wlodarczyk et al
[43]
have published a detailed account of the teratogenicities of individual antiepileptic drugs up to 2012, while Perruca[50]
authored a helpful, though now dated, review.
Several matters need to be appreciated in attempting to interpret the available data on antiepileptic drug-associated human teratogenesis. The overall foetal malformation rate associated with intrauterine antiepileptic drug exposure has been some two or three times that of the expected background normal population rate, which is believed to be somewhere in the range of 2–3% of all healthy pregnancies[5]
. Once pregnancies associated with exposure to the definite teratogen valproate are excluded from consideration, the residual malformation rate associated with antiepileptic drug exposure is probably little more than 1-2% greater than the rate for the general population. As a result, quite large data sets are usually required to obtain the statistical power needed to establish teratogenesis with reasonable probability in relation to the other antiepileptic agents. It is difficult for individual investigators to amass sufficiently large data sets to provide this level of evidence. Unfortunately, combining individual datasets from different sources in meta-analyses may result in new uncertainties in interpretation because inconsistencies in collection policies will probably be carried over into the meta-analyses. There is the question of how adequately each dataset represents the situation of the general population of pregnant women with epilepsy from which it is drawn. For instance, the Australian Pregnancy Register, with its policy of voluntary patient recruitment, has been estimated to capture only 8.7% of the expected pregnancies of Australian women with epilepsy[51]
. Comparable information from other pregnancy registers is not readily available. The individual datasets may not have employed identical definitions of what constitutes a foetal malformation, or a major congenital one, and their incidences of malformations may have been determined at different times after childbirth (almost 30%[52]
or 40%[5]
of malformations that can be identified one year after birth are not noticed if the assessment has occurred in the first postnatal days); malformation rates may have been expressed against various denominators (e.g. against live births), thus excluding more severe foetal malformations that have been managed by therapeutic abortion but including multiple progeny of pregnancies, or against numbers of pregnancies, which should have treated multiple births as though the pregnancy produced only a single offspring. A greater problem arises from the difficulties in collecting sufficiently-sized appropriate comparator populations to obtain the statistical power desirable for demonstrating increased foetal malformation rates relative to the normal or other comparator population. Comparisons between malformation rates associated with particular antiepileptic drugs are feasible, with the interpretation of the findings sometimes based on the hopeful assumption that the lowest malformation rate will not be lower than that in the normal population. From the standpoint of assessing the role of antiepileptic drugs in the situation, it would be desirable for comparator populations to comprise pregnant women with untreated epilepsy. There certainly are some women who elect to cease antiepileptic drugs in preparation for pregnancy, believing that this is desirable for the welfare of their foetuses, but this is unusual. If they could be collected in sufficient number, their earlier antiepileptic drug untreated pregnancies, if they existed, would provide an even better comparator. The use of such comparator data would tend to cancel out the consequences of any genetic tendency to foetal malformation. At least two studies have provided evidence that, if an antiepileptic drug-treated woman gives birth to a first child with a malformation, her subsequent babies are at increased risk of malformations compared with the babies of otherwise similar women whose first babies are not malformed[53],[54]
.
The above considerations apply in particular to pregnancy register data. Their existence makes comparisons between the findings from the different registers somewhat uncertain. If official institutional or governmental data repositories are used to provide large-scale, whole population comparator malformation rates, these rates have nearly always been derived from malformations detected at or very soon after the time of birth. They fail to include both malformations noticed after the baby involved has left hospital and pregnancies terminated because of detected foetal malformation. These various considerations almost certainly contribute to the otherwise surprisingly wide range of published malformation rate values that have been used to calculate relative risks for antiepileptic drug-associated malformations.
The material discussed so far has been based mainly on the outcomes of foetal exposure to single antiepileptic drugs during pregnancy. At least in contemporary clinical practice in Australia, some 20% of all pregnancies in women with epilepsy appear to be treated with combinations of two, or occasionally more, antiepileptic drugs[51]
. A similar figure is likely to apply elsewhere. A number of earlier publications found that, with antiepileptic drug combinations, foetal malformation rates were higher than when single antiepileptic drugs were used[38],[43],[55]
,[56]
. This resulted in advice being given that, as far as possible, antiepileptic drug polytherapy during pregnancy should be avoided. However, subsequent investigations found that the increased malformation rates in antiepileptic drug polytherapy occurred only when valproate, an agent with established teratogenicity, was one of the drugs involved in the combinations [57],[58],[59]
. More recently, with the increasing prescription of topiramate in pregnant women with epilepsy, the data of the Australian Pregnancy Register suggest that this drug, when used in combination with other antiepileptic agents, also can be associated with dose-related teratogenesis though, as mentioned above, at least in that data set, the drug did not appear to be a teratogen when used as the single antiepileptic agent[37]
. The literature does not seem to contain results of similar analyses based on material from other databases. There are as yet unpublished indications in the data of the Australian Register that carbamazepine, which in monotherapy is associated with statistically significant dose-related teratogenesis, seems to lose this capacity when prescribed in combination with another antiepileptic drug.
There are some aspects of antiepileptic drug-related teratogenicity that invite speculation: unlike classical human teratogens, such as thalidomide, individual antiepileptic drug teratogenicity is not restricted to particular organs and tissues; in spite of this, valproate exhibits a degree of dose-related tissue specificity in its associated malformation pattern; the pattern of valproate metabolism is dose-related[60],[61]
, with glucuronidation coming to preponderate over fatty acid β-oxidation as dose increases; dose-related malformations appear to be associated with topiramate use in polytherapy (when the drug’s clearance is increased[62]
and circulating topiramate concentrations would be expected to fall), but not in monotherapy. Whether or not the parent drugs are teratogens, it seems possible that some of the various metabolites of these drugs may interfere with the normal development of different foetal tissues and organs.
It has been known for some time that the prescription of folic acid before and during pregnancy can reduce the incidence of neural tube defects in the offspring of normal women[63],[64]
. Understandably, it was hoped that prescribing this substance prior to and during early pregnancy would also reduce the hazard of antiepileptic drug-associated foetal malformations in women with epilepsy. Unfortunately, the evidence available shows that this does not happen. In fact, the outcome of a large study based on the UK Pregnancy Register[65]
found preconception folate administration was associated with a somewhat higher foetal malformation rate than purely post-conception administration. Nonetheless, pre-conception and post-conception folate use continues to be recommended. There is some evidence that its intake in pregnancy may lead to improved neurodevelopmental outcomes in childhood after in utero antiepileptic drug exposure[66]
.
On the basis of current information, it would seem that foetal safety will be significantly enhanced if valproic acid or its sodium salt is not taken by pregnant women, and also probably topiramate, particularly if it is employed in combination with another antiepileptic drug. There is already evidence of decreasing use of valproate, and of its use in lower dosages, in more recent Australian Pregnancy Register data[33]
. When considering foetal malformations, the most desirable antiepileptic agents appear to be levetiracetam and probably lamotrigine. Of course, other matters such as seizure control in pregnancy have to be considered, particularly if antiepileptic drug regimens have to be changed before, or during, early pregnancy.
The foetus in post-natal existence: vitamin K deficiency
There have been reports of a bleeding diathesis manifesting in the first couple of neonatal days in babies who had been exposed during pregnancy to antiepileptic drugs such as phenytoin and phenobarbitone. These drugs are inducers of CYP450 isoenzymes and it was thought that this induction caused increased metabolic inactivation of vitamin K, resulting in impaired vitamin K-catalysed synthesis of blood coagulation factors. On these grounds, vitamin K came to be more or less routinely given to women with antiepileptic drug-treated seizure disorders during childbirth, or given to their neonates immediately after birth. This course of action may have prevented a drug-associated bleeding problem, though Kaaja et al
[67]
and Harden et al
[68]
failed to find published evidence of increased bleeding in neonates exposed to inducing antiepileptic drugs during pregnancy and questioned the need to continue the practice. With the increasing replacement of these older antiepileptic agents with more recently introduced non-inducing drugs, such vitamin K supplementation during pregnancy may largely cease to be an issue, but the vitamin may be administered to neonates when indicated.
Continuing antiepileptic drug effects
If a mother taking an antiepileptic drug breastfeeds, the baby continues to receive amounts of the antiepileptic drug to which it was previously exposed in utero, since some of the maternal intake of the drug will be excreted into her breast milk. If she elects not to breastfeed her newborn, the infant is abruptly deprived of the presence of a substance that may have sedative effects. There is some risk of the baby then exhibiting temporary withdrawal irritability phenomena, though seizures are unlikely. The actual occurrence of such events has not often been recorded, but there is an instance in relation to maternal oxcarbazepine intake[69]
.
In general, antiepileptic drugs enter breast milk through a process of passive diffusion. These molecules achieve concentration equilibrium between maternal plasma water and the aqueous phase of milk. However, the total drug concentration in milk is likely to be substantially higher than this because the drug may bind to milk proteins and, if lipophilic, also dissolve in the lipid content of the milk, which varies from time to time. Values are available for the likely ranges of antiepileptic drug concentrations in milk relative to simultaneous concentrations in maternal plasma[70]
. In general, the milk concentration values will not be higher than the concentrations in maternal plasma. Occasionally, the total amount of drug ingested from milk, particularly phenobarbitone or primidone, may be sufficient to cause sedation and feeding difficulty in an infant, but for the most part such problems are unlikely. As the baby grows older, breast milk provides a progressively decreasing portion of its food intake. Accordingly, the baby will have its exposure to the drug reduced in a relatively gradual manner that is unlikely to lead to safety concerns.
It has been reported that unprovoked seizures occur statistically significantly more often in the offspring of mothers with epilepsy (the great majority of whom would presumably have been treated with antiepileptic drugs) than in the offspring of fathers with the same disorder[71]
. The explanation for this observation, and in particular whether it is related to antiepileptic drug exposure in utero or postnatally, is unclear, though the study design would tend to rule out genetic contributions to the difference.
Later intellectual development
Some earlier studies noted the presence of reduced psychomotor development in infants associated with previous intrauterine antiepileptic drug exposure, though others did not[72]
. It seems unlikely that maternal epilepsy per se during pregnancy would affect the intelligence of the offspring. Gedzelmand and Meador[73]
found the IQs of children born to mothers with untreated epilepsy were similar to those for the general population, though Adab et al.
[74]
some years earlier had observed that there were lowered verbal IQ values in the children of mothers who had experienced more than four generalised tonic-clonic seizures during pregnancy. More recently, the NEAD study followed British and North American babies for six years after birth[66],[75]
. Its findings, and those of several other studies[74],[76],[77]
, have shown that on appropriate testing, children exposed in utero to the commonly used antiepileptic agents phenytoin, carbamazepine, in some studies phenobarbitone, and in one study levetiracetam[78]
, have exhibited intelligence scores and neurodevelopmental values that do not differ significantly from those of the general childhood population of the same age. However, children previously exposed to valproate in utero have had reduced mean scores, scores for particular aspects of intellectual function such as language development[79]
, and other evidences of neurodevelopmental delay.
Recent data have begun to provide evidence that breastfeeding by mothers taking antiepileptic drugs is also safe from the viewpoint of the intellectual development of the offspring, so long as valproate intake is not involved[80]
.
Reports have appeared of increased prevalences of social and behavioural disorders in children with intrauterine exposure to antiepileptic drugs[81]
. Some of the affected children have met the diagnostic criteria of autism spectrum disorder or autism itself. In several studies, valproate has been found to be the drug particularly associated with such autism[82],[83],[84],[85]
.
These findings in relation to valproate further emphasise the desirability of avoiding the drug’s use in women with epilepsy or with other disorders where there is a possibility that pregnancy may occur while they are taking the drug. There is some evidence that these intellectual and behavioural disturbances in childhood are valproate dose related, but it is not known when, during the development of the foetal or possibly the postnatal brain, exposure to the drug produces these harmful effects. This is unlike the situation in relation to foetal malformation, which develops in the first trimester of pregnancy. Consequently, the belief can no longer be sustained that, if valproate was avoided for the first three or four months of pregnancy, it is safe to resume the intake of the drug in the latter half of pregnancy.
Antiepileptic drug use for indications other than epilepsy
Data on the interaction between antiepileptic drugs and pregnant women with epilepsy have provided nearly all the knowledge-base that underlies the use of these drugs in other disorders in pregnancy. The question of safety of these drugs in other disorders not only has to take into consideration the natural histories of these disorders during the course of pregnancy, but also the degree of likely psychological and physical harm that could result from loss of symptom control, or from becoming aware of carrying or giving birth to a malformed foetus. For instance, if valproate was being used as a migraine preventative, the drug could usually be withdrawn prior to a planned pregnancy with little more than temporary consequences because migraine often tends to become inactive as pregnancy progresses. On the other hand, if the same drug was being used to successfully suppress a psychiatric disorder in which suicide was a distinct possibility, the decision to withdraw the drug would involve careful consideration. Relapse of psychiatric disorders following antiepileptic drug withdrawal during pregnancy has been described[86]
. There are too many possible scenarios for further consideration here.
Conclusion
If antiepileptic drug dosages in pregnant women with epilepsy are adjusted carefully and at frequent enough intervals during pregnancy and the postnatal weeks, the adjustments being guided whenever feasible by plasma antiepileptic drug concentration monitoring, the risk of maternal harm from loss of seizure control or from drug overdosage should be reduced, so long as the pregnant woman complies with the dosage recommendations. Such management is likely to prove more satisfactory if full, or best achievable, seizure control is obtained before pregnancy commences. There are data from more than one source indicating that, if seizures are already controlled for 9 or 12 months before pregnancy begins[87]
,[88]
, there is a substantially reduced risk of seizure recurrence during pregnancy, thus lessening the risk of harm to the mother and, less probably, to her foetus.
Such appropriate application of existing, mainly pharmacokinetic, knowledge may have provided a significant beneficial effect in relation to the safety of antiepileptic drug therapy from the standpoint of epileptic seizure control during pregnancy. However, the situation is rather different in relation to the occurrence of foetal malformations and the intellectual and neurodevelopmental shortcomings related to maternal intake of the drugs, particularly valproate. It may be simple enough to advise that valproate should not be used in women capable of becoming pregnant. This advice may be reasonably realistic in relation to focal epilepsies, where there are several alternative antiepileptic agents of more or less equal efficacy and with substantially greater safety from the standpoint of foetal malformation, and also probably from that of the intellectual and behavioural development of the foetus and infant. However, in the case of primary generalised epilepsies, valproate is usually the most effective available agent. Its potential alternatives tend to fall short in maintaining seizure control. Therefore, replacing valproate in anticipation of pregnancy, or during pregnancy, may cause harm to the pregnant woman. The advice is sometimes given that, if there is no better alternative in these circumstances, valproate should be prescribed in the minimal effective dose. First, finding such a dose will usually involve a process of cautious downward dose titration over a period of time to determine the drug’s therapeutic threshold in the individual, which may result in the loss of seizure control with its attendant disadvantages. Second, present-day knowledge does not provide assurance that the lowest effective valproate dose from the point of view of seizure control will be safe from avoiding disturbed development of intellectual function in the child. Further, the generalised epilepsies in which valproate is required in particular women because other drugs have proved inadequate are often those in which high valproate doses are needed. Currently there seems no totally satisfactory way of resolving this difficulty. A better solution would require the availability of a newer antiepileptic drug with a high degree of efficacy in primary generalised epilepsy, a low incidence of adverse effects, and a considerable degree of safety in respect to foetal malformation and altered intellectual development.
Acknowledgements
The author is grateful for access to the data of the Australian Register of Antiepileptic Drugs in pregnancy. He has no conflicts of interest, financial or otherwise, in writing this review.
Financial and conflicts of interest disclosure
The author has no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilised in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Bobo WV, Davis RL, Toh S et al. Trends in the use of antiepileptic drugs among pregnant women in the US, 2001-2007: a medication exposure in pregnancy risk evaluation program study. Paediatr Perinat Epidemiol 2012;26:578–588. doi: 10.1111/ppe.12004
[2] Schmidt D. The effect of pregnancy on the natural history of epilepsy: review of the literature. In: Janz D, Bossi L, Dam M, Helge H, Richens A, Schmidt D (eds.) Epilepsy, pregnancy, and the child. Raven Press: New York 1982;3–14.
[3] Knight AH & Rhind EG. Epilepsy and pregnancy: a study of 153 pregnancies in 59 patients. Epilepsia 1975;16:99–110. doi: 10.1111/j.1528-1157.1975.tb04726.x
[4] Schmidt D, Canger R, Avanzini G et al. Change in seizure frequency in pregnant epileptic women. J Neurol Neurosurg Psychiatry 1983;46:751–755. doi: 10.1136/jnnp.46.8.751
[5] Battino D & Tomson T. Management of epilepsy during pregnancy. Drugs 2007;67:2727–2746. doi: 10.2165/00003495-200767180-00007
[6] Edey S, Moran N & Nashef L. SUDEP and epilepsy-related mortality in pregnancy. Epilepsia 2013;55:72–74. doi: 10.1111/epi.12621
[7] Dam M, Mygind KI & Christiansen J. Antiepileptic drugs: plasma clearance during pregnancy. In: Janz D (ed.) Epileptology. Thieme: Stuttgart 1976;179–183.
[8] Lander CM, Edwards VE, Eadie MJ et al. Plasma anticonvulsant concentrations during pregnancy. Neurology 1977;27:128–131. doi: 10.1212/WNL.27.2.128
[9] Bernus I, Hooper WD, Dickinson RG et al. Metabolism of carbamazepine and co-administered anticonvulsants during pregnancy. Epilepsy Res 1995;21:65–75. doi: 10.1016/0920-1211(95)00012-Y
[10] Pennell PB, Peng L, Newport DJ et al. Lamotrigine in pregnancy: clearance, therapeutic drug monitoring, and seizure frequency. Neurology 2008;70:2130–2136. doi: 10.1212/01.wnl.0000289511.20864.2a
[11] Sabers A & Petrenaite V. Seizure frequency in pregnant women treated with lamotrigine monotherapy. Epilepsia 2009;50:2163–2166. doi: 10.1111/j.1528-1167.2009.02166.x
[12] Pirie DAJ, Wattar BHA, Pirie AM et al. Effect of monitoring strategies on seizures in pregnant women on lamotrigine: a meta-analysis. Europ J Obstet Gynec Reproduct Biol 2013;172:26-31. doi: 10.1016/j.ejogrb.2013.10.021
[13] Vajda F, O’Brien T, Graham J et al. Does pregnancy per se make epilepsy worse? Acta Neurol Scand 2015. doi: 10.1111/ane.12479
[14] Janz D & Fuchs U. Are anti-epileptic drugs harmful when given during pregnancy? Ger Med Mon 1964;9:20–22.
[15] Speidel BD & Meadow SR. Maternal epilepsy and abnormalities of the fetus and newborn. Lancet 1972;2:839–843. doi: 10.1016/S0140-6736(72)92209-X
[16] Annegers JF, Baumgartner KB, Hauser WA et al. Epilepsy, antiepileptic drugs, and the risk of spontaneous abortion. Epilepsia 1988;29:451–458. doi: 10.1111/j.1528-1157.1988.tb03745.x
[17] Richmond JR, Krishnamoorthy P, Andermann E et al. Epilepsy and pregnancy: an obstetric perspective. Am J Obstet Gynecol 2004;190:371–379. doi: 10.1016/j.ajog.2003.09.020
[18] Katz O, Levy A, Wiznitzner A et al. Pregnancy and perinatal outcome in epileptic women: a population-based study. J Matern Fetal Neonatal Med 2006;19:21–25. doi: 10.1080/14767050500434096
[19] McPherson JA, Harper LM, Odibo AO et al. Maternal seizure disorder and risk of adverse pregnancy outcomes. Am J Obstet Gynecol 2013;208:378.e1-5. doi: 10.1016/j.ajog.2013.01.048
[20] Kilic D, Pedersen H, Kjaersgaard MI et al. Birth outcomes after prenatal exposure to antiepileptic drugs — a population-based study. Epilepsia 2014;55:1714–1721. doi: 10.1111/epi.12758
[21] Bech BH, Kjaersgaard MI, Pedersen HS et al. Use of antiepileptic drugs during pregnancy and risk of spontaneous abortion and stillbirth: population based cohort study. Br Med J 2014;349:g5159. doi: 10.1136/bmj.g5159
[22] Borthen I & Gilhus NE. Pregnancy complications in patients with epilepsy. Curr Opin Obstet Gynecol 2012;24:78–83. doi: 10.1097/GCO.0b013e32834feb6a
[23] Rauchenzaue M, Ehrensberger M, Prieschl M et al. Generalised tonic-clonic seizures and antiepileptic drugs during pregnancy: a matter of importance for the baby? J Neurol 2013;260:484–488. doi: 10.1007/s00415-012-6662-8
[24] Veiby G, Daltveit AK, Engelsen BA et al. Pregnancy, delivery, and outcome for the child in maternal epilepsy. Epilepsia 2009;50:30–39. doi: 10.1111/j.1528-1167.2009.02147.x
[25] Pennell PB, Klein AM, Browning N et al. Differential effects of antiepileptic drugs on neonatal outcomes. Epilepsy Behav 2012;24:449–456. doi: 10.1016/j.yebeh.2012.05.010
[26] Artama M, Gissler M, Malm L et al; the Drug and Pregnancy Group. Effects of maternal epilepsy and antiepileptic drugs during pregnancy on personal health of offspring: national retrospective cohort study in Finland. Drug Saf 2013;36:359–369. doi: 10.1007/s40264-013-0052-8
[27] Barroso FV, Araujo Júnior E, Guazelli CA et al. Perinatal outcomes from the use of antiepileptic drugs during pregnancy: a case-control study. Matern Fetal Neonatal Med 2014;10:1–6. doi: 10.3109/14767058.2014.955006
[28] Di Liberti JH, Farndon RA, Dennis NR et al. The fetal valproate syndrome. Am J Med Genet 1984;19:473–481. doi: 10.1002/ajmg.1320190308
[29] Kaneko S, Battino D, Andermann E et al. Congenital malformations due to antiepileptic drugs. Epilepsy Res 1999;33:145–158. doi: 10.1016/S0920-1211(98)00084-9
[30] Samrén EB, van Duijn CM, Lieve Christiaens GCM et al. Antiepileptic drug regimens and major congenital abnormalities in the offspring. Ann Neurol 1999;6:739–746. doi: 10.1002/1531-8249(199911)46:5<739::AID-ANA9>3.0.CO;2-2
[31] Vajda FJ, O’Brien TJ, Hitchcock A et al. Critical relationship between sodium valproate dose and human teratogenicity: results of the Australian register of anti-epileptic drugs in pregnancy. J Clin Neurosci 2004;11:854-858. doi: 10.1016/j.jocn.2004.05.003
[32] Omtzigt JG, Los FJ, Grobbee DE et al. The risk of spina bifida aperta after first-trimester exposure to valproate in a prenatal cohort. Neurology 1992;42(4):119–125. PMID: 1574165
[33] Vajda FJE, O’Brien TJ, Graham J et al. Dose dependence of fetal malformations associated with valproate. Neurology 2013;81:999–1003. doi: 10.1212/WNL.0b013e3182a43e81
[34] Miki T, Reo T, Tohru K et al. Risks of congenital malformations in offspring exposed to valproic acid in utero: emergence of the signals over the last 30 years. J Popul Ther Clin Pharmacol 2014;21:2(e277).
[35] Holmes LB & Hernandez-Diaz S. Newer anticonvulsants: lamotrigine, topiramate and gabapentin. Birth Defects Res (Part A) 2012;94:599–606. doi: 10.1002/bdra.23028
[36] Marguilis AV, Mitchell AA & Gilboa SM. Use of topiramate in pregnancy and risk of oral clefts. Am J Obstet Gynec 2014;207:e1–7.
[37] Vajda FJ, O’Brien TJ, Lander CM et al. The teratogenicity of the newer antiepileptic drugs — an update. Acta Neurol Scand 2014;130:234–238. doi: 10.1111/ane.12280
[38] Samrén EB, van Duijn CM, Koch S et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia 1997;38:981–990. doi: 10.1111/j.1528-1157.1997.tb01480.x
[39] Matalon S, Schectman S, Goldzweig G et al. The teratogenic effect of carbamazepine: a meta-analysis of 1255 exposures. Reprod Toxicol 2002;16:9–17. doi: 10.1016/S0890-6238(01)00199-X
[40] Vajda FJE, O’Brien TJ, Graham J et al. Is carbamazepine a human teratogen? J Clin Neurosci 2016;23:34–47. doi: 10.1016/j.jocn.2015.07.011
[41] Holmes LB, Wyszynski DF & Lieberman E. The AED (antiepileptic drug) pregnancy registry: a 6-year experience. Arch Neurol 2004;61:673–678. PMID: 15148143
[42] Tomson T, Battino D, Bonizzoni E et al. Dose-dependent risk of malformation with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol 2011;10:609–617. doi: 10.1016/S1474-4422(11)70107-7
[43] Wlodarczyk BJ, Palacios AM, George TM et al. Antiepileptic drugs and pregnancy outcomes. Am J Med Genet A 2012;158A:2071–2090. doi: 10.1002/ajmg.a.35438
[44] Morrow J, Russell A, Guthrie E et al. Malformation risk of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 2006;77:193–198. doi: 10.1136/jnnp.2005.074203
[45] Campbell E, Kennedy F, Russell A et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry 2014;85:1029–1034. doi: 10.1136/jnnp-2013-306318
[46] Mahwinney E, Craig J, Morrow J et al. Levetiracetam in pregnancy. Results from the UK and Ireland epilepsy and pregnancy registers. Neurology 2013;80:401–405. doi: 10.1212/wnl.0b013e31827f0874
[47] Veiby G, Daltveit AK, Engelsen BA et al. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol 2014;261:579–588. doi: 10.1007/s00415-013-7239-x
[48] Chaudhry SA, Jong G & Koren G. The fetal safety of levetiracetam: a systematic review. Reprod Toxicol 2014;46:40–45. doi: 10.1016/j.reprotox.2014.02.004
[49] Guttuso T Jr, Shaman M & Thornburg LL. Potential maternal symptomatic benefit of gabapentin and review of its safety in pregnancy. Eur J Obstet Gynecol Reprod Biol 2014;181:280–283. doi: 10.1016/j.ejogrb.2014.08.013
[50] Perucca E. Birth defects after prenatal exposure to antiepileptic drugs. Lancet Neurol 2005;4:781–786. doi: 10.1016/S1474-4422(05)70224-6
[51] Vajda FJE, O’Brien TJ, Graham J et al. The Australian register of antiepileptic drugs in pregnancy: changes over time in the epileptic population. J Clin Neurosci 2014;21:1478–1482. doi: 10.1016/j.jocn.2013.11.049
[52] Vajda FJE, O’Brien T, Hitchcock A et al. The Australian antiepileptic drug in pregnancy register: aspects of data collection and analysis. J Clin Neurosci 2007;14:936–942. doi: 10.1016/j.jocn.2006.08.015
[53] Campbell E, Devenney E, Morrow J et al. Recurrence risk of congenital malformations in infants exposed to antiepileptic drugs in utero. Epilepsia 2013;54:165–171. doi: 10.1111/epi.12001
[54] Vajda FJE, O’Brien TJ, Lander CM et al. Tetratogenesis in repeated pregnancies in antiepileptic drug-treated women. Epilepsia 2013;54:181–186. doi: 10.1111/j.1528-1167.2012.03625.x
[55] Nakane Y, Okuma T, Takahashi R et al. Multi-institutional study on the teratogenicity and fetal toxicity of antiepileptic drugs: a report of a collaborative study group in Japan. Epilepsia 1980;21:663–680. doi: 10.1111/j.1528-1157.1980.tb04320.x
[56] Lindhout D & Omtzigt JG. Pregnancy and the risk of teratogenicity. Epilepsia 1992;33(4):S41–S48. doi: 10.1111/j.1528-1157.1992.tb06226.x
[57] Vajda F, Hitchcock A, Graham J et al. The teratogenic risk of antiepileptic drug polytherapy. Epilepsia 2010;51:805–810. doi: 10.1111/j.1528-1167.2009.02336.x
[58] Mawer G, Briggs M, Baker GA et al. Pregnancy with epilepsy: obstetric and neonatal outcome of a controlled study. Seizure 2010;19:112–119. doi: 10.1016/j.seizure.2009.11.008
[59] Holmes LB, Mittendorf R, Shen A et al. Fetal effects of anticonvulsant polytherapies. Arch Neurol 2011;68:1275–1281. doi: 10.1001/archneurol.2011.133
[60] Dickinson RG, Hooper WD, Dunstan PR et al. Urinary excretion of valproate and some metabolites in chronically treated patients. Ther Drug Monit 1989;11:127–133. doi: 10.1097/00007691-198903000-00002
[61] Eadie MJ, McKinnon GE, Dunstan PR et al. Valproate metabolism during hepatotoxicity associated with the drug. Quart J Med (NS) 1990;77:1229–1240. doi: 10.1093/qjmed/77.3.1229
[62] Britzi M, Perucca E, Soback S et al. Pharmacokinetic and metabolic investigation of topiramate disposition in healthy subjects in the absence and in the presence of enzyme induction by carbamazepine. Epilepsia 2005;46:378–384. doi: 10.1111/j.0013-9580.2005.55204.x
[63] Champel V, Radal M, Moulin-Vallez M et al. Should folic acid be given to women treated with valproic acid and/or carbamazepine? Folic acid and pregnancy in epilepsy. Rev Neurol Paris 1999;155:220–224.
[64] Berry RJ, Li Z, Erickson JD et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med 1999;341:1485–1490. Erratum in N Engl J Med 1999;341:1864. doi: 10.1056/NEJM199911113412001
[65] Morrow JI, Hunt SJ, Russell AJ et al. Folic acid use and major congenital malformations in offspring of women with epilepsy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 2009;80:506–511. doi: 10.1136/jnnp.2008.156109
[66] Meador KJ, Baker GA, Browning N et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 22009;360:1597–1605. doi: 10.1056/NEJMoa0803531
[67] Kaaja E, Kaaja R, Matila R et al. Enzyme-inducing antiepileptic drugs in pregnancy and the risk of bleeding in the neonate. Neurology 2002;58:549–553. doi: 10.1212/WNL.58.4.549
[68] Harden CL, Pennell PB, Koppel BS et al. Management issues for women with epilepsy — focus on pregnancy (an evidence-based review): III. Vitamin K, folic acid, blood levels, and breast-feeding: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia 2009;50:1247–1255. doi: 10.1111/j.1528-1167.2009.02130.x
[69] Rolnitsky A, Merlob P & Klinger G. In utero oxcarbazepine and a withdrawal syndrome, anomalies, and hyponatremia. Pediatr Neurol 2013;48:466–468. doi: 10.1016/j.pediatrneurol.2013.02.012
[70] Davanzo R, Bo SD, Bua J et al. Antiepileptic drugs and breastfeeding. Ital J Pediatr 2013;39:50. doi: 10.1186/1824-7288-39-50
[71] Ottman R, Annegers JF, Hauser WA et al. Higher risk of seizures in offspring of mothers than of fathers with epilepsy. Am J Hum Genet 1988;43:257–264.
[72] Deblay MF, Vert P & André M. [Children of epileptic mothers]. Nouv Presse Med 1982;11:173–176.
[73] Gedzelman E & Meador KJ. Aeds in women with epilepsy during pregnancy. Ther Adv Drug Saf 2012;3:71–87. doi: 10.1177/2042098611433192
[74] Adab N, Kini U, Vinten J et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry 2004;75:1575–1583. doi: 10.1136/jnnp.2003.029132
[75] Meador KJ, Baker GA, Browning N et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 2013;12:244–252. doi: 10.1016/S1474-4422(12)70323-X
[76] Gaily E, Kantola-Sorsa F, Hiilesmaa V et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology 2004;62:28–32. doi: 10.1212/WNL.62.1.28
[77] Cummings C, Stewart M, Stevenson M et al. Neurodevelopment of children exposed to lamotrigine, sodium valproate and carbamazepine. Arch Dis Child 2011;96:643–647. doi: 10.1136/adc.2009.176990
[78] Shallcross R, Bromley RL, Irwin B et al. Child development following in utero exposure: levetiracetam vs sodium valproate. Neurology 2011;76:383–389. doi: 10.1212/WNL.0b013e3182088297
[79] Nadebaum C, Anderson VA, Vajda F et al. Language skills of school-aged children prenatally exposed to antiepileptic drugs. Neurology 2011;76:719–726. doi: 10.1212/WNL.0b013e31820d62c7
[80] Meador KJ, Baker GA, Browning N et al. Breastfeeding in children of women taking antiepileptic drugs: cognitive outcomes at age 6 years. JAMA Pediatr. 2014;168:729–736. doi: 10.1001/jamapediatrics.2014.118
[81] Rasalam AD, Hailey H, Williams JH et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol 2005;47:551–555. doi: 10.1017/S0012162205001076
[82] Christensen J, Grønborg TK, Sørensen MJ et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013;309:1696–1703. doi: 10.1001/jama.2013.2270
[83] Moore SJ, Turnpenny P, Quinn A et al. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet 2000;37:489–497. doi: 10.1136/jmg.37.7.489
[84] Williams G, King J, Cunningham M et al. Fetal valproate syndrome and autism: additional evidence of an association. Dev Med Child Neurol 2001;43:202–206. doi: 10.1111/j.1469-8749.2001.tb00188.x
[85] Bromley RL, Mawer G, Clayton-Smith J et al; Liverpool and Manchester Neurodevelopment Group. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology 2008;71:1923–1924. doi: 10.1212/01.wnl.0000339399.64213.1a
[86] Viguera AC, Whitfield T, Baldessarini RJ et al. Risk of recurrence in women with bipolar disorders during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry 2007;164:1817–1824. doi: 10.1176/appi.ajp.2007.06101639
[87] Vajda FJE, Hirchcock A, Graham J et al. Seizure control in antiepileptic drug-treated pregnancy. Epilepsia 2008;49:172–175. doi: 10.1111/j.1528-1167.2007.01412.x
[88] Harden C, Hopp J, Ting TY et al. Management issues for women with epilepsy — Focus on pregnancy (an evidence-based review): 1.Obstetrical complications and change in seizure frequency. Epilepsia 2009;50:1229–1236. doi: 10.1111/j.1528-1167.2009.02128.x
You may also be interested in

Epilepsy drugs levetiracetam and zonisamide should not be routinely used first line in the NHS, conclude researchers

No difference in cognitive outcomes in children born to women taking antiseizure medicines
