Abstract
Aim
To assess the accuracy with which community pharmacists are able to make a diagnosis of influenza.
Design
Cross-sectional study. Pharmacists’ diagnoses of influenza in patients presenting to the pharmacy were compared with results of nasopharyngeal swab polymerase chain reaction (PCR) viral tests.
Subjects and setting
22 community pharmacists in the UK.
Results
Out of 217 subjects, pharmacists diagnosed 53 with influenza and, of these, 27 were confirmed virologically (positive predictive value of 51%). A raised temperature measured at presentation did not improve diagnostic accuracy. 21 (40%) of the 53 diagnosed influenza cases and 69 (32%) of all 217 subjects presented within 48 hours of symptom onset. In subjects presenting within 48 hours, a total symptom score exceeding 10 (39 subjects) was associated with confirmed flu in 38%, compared with a total score less than 10 (30 subjects) associated with confirmed influenza in only 20%.
Conclusion
Despite a low level of circulating influenza over the study period, community pharmacists achieved a level of accuracy in diagnosing influenza (51%) which compares favourably with results achieved in clinical trials of neuraminidase inhibitors where recruitment was limited to early presentation. The data suggest that pharmacists could play an extended role in the management of seasonal influenza. Most patients did not present early enough to benefit from treatment with antiviral drugs. There is a need for better public understanding of the benefits of these drugs.
The availability of antiviral treatments for influenza, such as neuraminidase inhibitors (NIs), that demonstrate greatest benefit if administered within 48 hours of symptom onset provides a challenge to UK health care delivery systems. Evidence suggests that patients with influenza-like illness often do not present early enough to their GPs to obtain the potential benefit from treatment with these drugs.1 Furthermore, in a severe influenza epidemic, GPs may not have the resources to see and prescribe for all patients who need treatment.2
As part of the Government’s objective to improve patients’ choice and access to health care, there is interest in extending the clinical role of community pharmacists.3 Management of some acute self-limiting conditions has been shown to be successfully transferred from the GP to the community pharmacist, with pharmacy care reducing GP workload and being seen by patients as providing effective and convenient access.4–6 Influenza is a self-limiting condition but it can be associated with serious complications especially in at-risk groups and the elderly. Community pharmacists could potentially initiate antiviral drug treatment of influenza.
To investigate the feasibility of wider community pharmacist involvement in the care of patients with influenza, I was interested to assess pharmacists’ ability to diagnose influenza. I have not found any published evidence on this. Large-scale community-based clinical trials with NI antivirals have demonstrated a diagnostic accuracy, or positive “pick up” rate, of 58–79 per cent.7–9 In most of these, the recruitment of cases of clinical influenza was based on a combination of at least one respiratory symptom (cough, sore throat or runny nose), at least one constitutional symptom (headache, malaise, myalgia, sweats, chills or fatigue) and a raised temperature (>37.8C). Recruitment was restricted to patients presenting early in the course of the disease. The aims of this study were:
- To investigate the level of accuracy of diagnosis of influenza in the community pharmacies
- To assess the usefulness of a simple symptom-based questionnaire as an aid to accurate diagnosis
Methods
The study was undertaken during the influenza seasons of 2003/04 and 2004/05. Independent community pharmacists located throughout the UK were selected as study sites. These 22 pharmacists were chosen from a list of registered pharmacists who were contacted by letter with a covering endorsement from the National Pharmacy Association. Selection criteria included the pharmacist’s willingness to participate and suitable facilities in the pharmacy for the study (ie, adequate staffing and presence of a quiet area with a sink where the patient could be assessed and a nasopharyngeal swab taken). Study pharmacists were given training on the technique for taking swabs.
Surveillance in the community by the Royal College of General Practitioners and the Health Protection Agency suggested an increase influenza virus activity in the community.10 Pharmacy posters and leaflets were used to draw the attention of potential subjects to the study. To minimise the potential for selection bias, pharmacy staff were instructed not to be selective in their approach to potential subjects. The study was open to all patients aged 12 years or over.
Patients who expressed an interest in the study were shown copies of the study patient information leaflet explaining the purpose of the study. Those who wished to participate were given a consent form to read and sign.
The patients were then asked to complete a short questionnaire and the pharmacist asked them about their symptoms (including fever, cough, stuffy nose, sore throat, headache, fatigue and muscle weakness) scoring each according to severity (absent=0, mild=1, moderate=2, severe=3). The patient’s temperature was measured using an oral digital electronic thermometer. The pharmacist then recorded his or her opinion on the diagnosis. This judgement was made on the basis of the information available, including the symptoms reported and the measured oral temperature, but also on comments made by the patient regarding close contacts who were ill and local information acquired from routine pharmacy practice. After assessing a patient, a nasopharyngeal swab was taken, inserted in the medium provided and sent to the Health Protection Agency (HPA) for examination by polymerase chain reaction (PCR).11 The completed questionnaires (case report forms) were sent to the research team for analysis. The pharmacist followed usual practice for responding to the patient’s symptoms. No treatment was involved in the study. Pharmacists were paid a fee of £15 per recruited patient as a reimbursement for the time involved.
A copy of the HPA report was sent to the lead investigator and to the pharmacist and to the patient’s general practitioner when positive. Study data were entered on an Excel spread sheet for analysis in accordance with the protocol. Ethics approval for the study was given by West Midlands Multi-centre Research Ethics Committee.
The primary study assessment was a comparison of the pharmacist’s diagnosis of influenza against the PCR virology results, using standard efficacy parameter tests. With an expected positive predictive value of 60 per cent, a two-sided 95 per cent confidence interval would have a range of 10 per cent with a sample size of 369.
Results
The study saw 15 pharmacists recruit 217 patients. Two further patients were recruited but had no swab results and were excluded from the analyses. One pharmacist recruited 159 patients (73 per cent of the total), three pharmacists recruited six patients each and a further seven pharmacists recruited one or two patients each.
Patient questionnaire
The ages of the 217 patients recruited ranged from 12–83 years (mean 41 years). The responses to the questionnaire are shown in Table 1 (percentages based on total answers received).
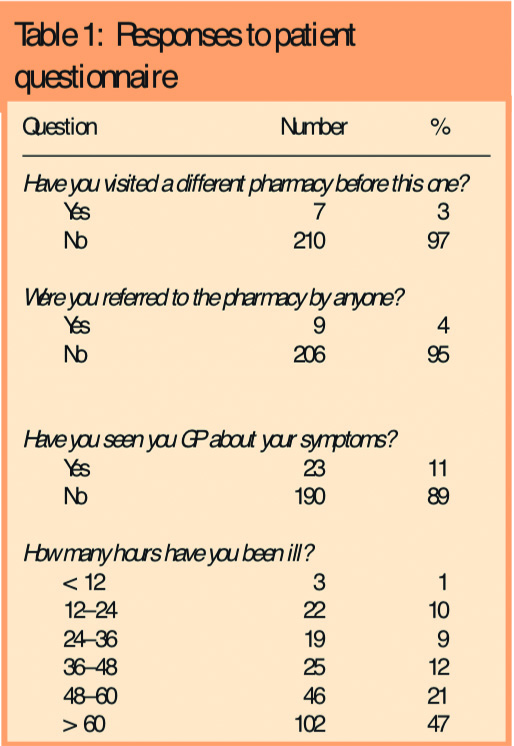
They indicated that 23 patients (11 per cent) had previously seen a GP about their symptoms. Only two patients reported that they had taken their temperature before presenting to the pharmacist (a further 10 patients did not answer this question). Almost half the patients (47 per cent) had been ill for more than 60 hours before coming to the pharmacy and a third (32 per cent) presented within 48 hours of symptom onset.
Of the 217 cases presenting with symptoms suggestive of influenza, 205 reported fever, 209 cough, 215 a stuffy nose and sore throat, 198 headache, 203 fatigue and 191 muscle ache or weakness. The grading of symptoms according to severity as reported by the patient is given in Table 2.
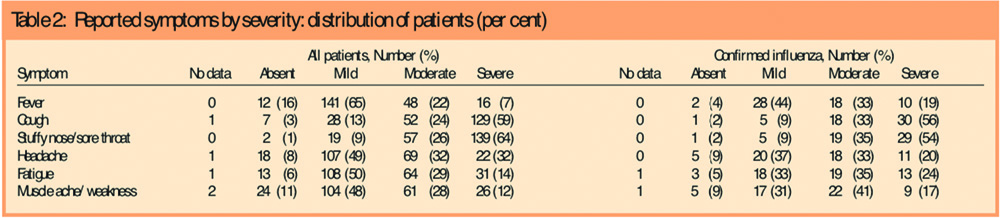
In summary, cough, and a stuffy nose and sore throat were predominantly described as severe and other symptoms as mild or moderate. The symptom profile of influenza PCR-positive subjects was little different from that of all patients, although PCR-positive patients were more likely to have reported feverishness and less likely to have reported a stuffy nose and sore throat as moderate or severe. An additional analysis restricted to patients diagnosed by the pharmacist and PCR-positive further emphasised this tendency.
The total symptom score for each of the 217 subjects was calculated from their graded individual symptom scores. The mean score was 10.6 (standard deviation 2.7). For the total 54 PCR-positive subjects it was 11.5 (SD 2.9), for the 163 PCR-negative subjects it was 10.3 (SD 2.6). For the 27 pharmacist-positive and PCR-positive subjects it was 13.1 (SD 2.3) and for the 26 pharmacist-positive and PCR-negative subjects it was 12.3 (SD 2.6). Although these differences are small, they are consistent with influenza being a more severe illness than the other similar illnesses which prompt consultation with pharmacists. This conclusion was reinforced in a separate analysis of 69 subjects presenting within 48 hours of symptom onset. Of these, 30 reported a total symptom score of less than 10 and the probability of confirmed influenza was 20 per cent: 39 reported a score of more than 10 and the equivalent probability was 38.5 per cent. However, all these results should be seen against a background of wide variability.
Of the total 217 subjects, 53 (24 per cent) were provisionally diagnosed with influenza following the interview with the pharmacist and, among the 69 presenting within 48 hours of symptom onset, 25 (36 per cent) were provisionally diagnosed. The overall infection rate according to the PCR results was 25 per cent (54 of 217). The data on pharmacist diagnosis and the result of PCR investigation of the nasopharyngeal specimen were analysed to calculate diagnostic efficacy statistics.
The positive predictive value (expressed as a ratio) of the pharmacist’s diagnosis was 0.51 (with a confidence interval of 0.33–0.64). The results of secondary analyses of diagnostic accuracy restricted to patients with a raised temperature (>37.8C) and according to the duration of symptoms at presentation (< or > 48 hours) are given in Table 3.
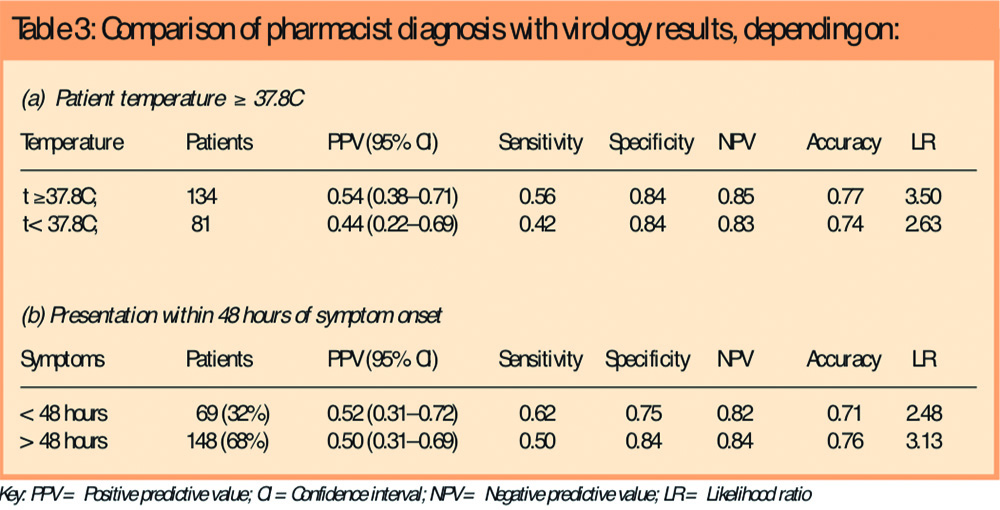
When measured by the pharmacist, 134 subjects (62 per cent) had a raised temperature but the diagnostic efficacy statistics were scarcely different from those in whom the temperature was not raised. Neither was there much difference between these statistics as calculated in subjects consulting in the first 48 hours compared with those consulting later.
Since recruitment to this study was dominated by a single pharmacist we analysed the data for this pharmacist separately from the other 10 pharmacists. The PPV based on recruitment in this pharmacy was 0.48 (CI 0.31–0.66) and similar to that based on the remaining pharmacies, 0.55 (CI 0.34-0.75); values for specificity and sensitivity were also similar.
Discussion
Comparison of the pharmacists’ diagnosis of influenza against confirmed virological (PCR) results from nasopharyngeal swabs demonstrated a PPV or “pick-up” rate of 51 per cent. Although recruitment was heavily biased towards one particular pharmacist, the results from this source were similar to those in the 10 other pharmacies.
The diagnosis of influenza-like illness is based principally on clinical symptoms assisted by knowledge of the presence of influenza in the community.10–13 The symptoms of influenza are not specific, but when influenza virus is circulating in the community it is more likely that a person with influenza-like illness has true influenza. Nevertheless, in this study, half the persons diagnosed with influenza by PCR were not diagnosed by the pharmacist. It is well known that persons with influenza do not always present with the classical signs associated with this virus.14 Levels of influenza circulating in the community in both these winters have been much lower than in recent years15 and therefore the 51 per cent positive pick-up rate is encouraging. It demonstrates what can be achieved in everyday pharmaceutical practice and is comparable to that achieved in primary care and not far short of case ascertainment in large-scale influenza trials (58 to 79 per cent7–9) conducted in winters of higher influenza activity, based on strict diagnostic criteria and where recruitment has been restricted to presentation within 48 hours of symptom onset. In this study, separate analysis according to the pharmacist recorded temperature at presentation did not show significantly improved diagnosis with raised temperature. The inclusion of 47 per cent of cases presenting more than 60 hours since illness onset may account for this surprising finding. It was interesting to notice how few patients had actually taken their temperature before seeing the pharmacist.
Most patients had not seen their GP and had come to the pharmacy direct. However, as has also been reported from a practice-based study, less than one-third of patients presented within 48 hours of symptom onset and almost half presented more than 60 hours later.1 A perception that there is no effective treatment available, a widespread stoical attitude towards influenza infection and a tradition of self-management all combine to limit the potential benefit that NI antivirals can bring to infected patients. In recent years, influenza has been comparatively mild compared with 30 years ago.15 Patients will have to be encouraged to seek treatment sooner if NI antivirals are to be deployed effectively whether in regular seasonal epidemics or in a pandemic.16
The National Institute for Health and Clinical Excellence (NICE) recommends use of neuraminidase inhibitors to treat influenza in at-risk adults if treatment can be started within 48 hours of symptom onset.10 This advice is based on the effectiveness of these drugs in typical recent seasonal influenza epidemics. Although this may not be the case with the introduction of a pandemic strain, the principle of earliest possible treatment is imperative.17 This principle presents a challenge to the NHS to achieve effective care in both pandemic and seasonal influenza and the greater involvement of community pharmacists should be considered.
The responsibility for the management of influenza in the community in the UK has largely been the remit of GPs. However, pharmacists are now being encouraged to take an expanded role in the management of both chronic and self-limiting conditions.3 At present, community pharmacist involvement in influenza management involves responding to symptoms, referral to a GP as necessary, and dispensing medicines. In Scotland, selected pharmacists now carry out influenza immunisation in accordance with group directive policy.18 There is also interest in supplying the NI oseltamivir via a patient group direction (PGD). More than 80 pharmacists in South Staffordshire have been trained to support rapid access to oseltamivir, with supply authorised through a PGD, should an influenza pandemic occur.2
A 50 per cent pick-up rate in a pharmacy setting is particularly significant in the context of the use of near patient tests for diagnosis. If clinical judgement in adults is already this predictable even at pharmacy presentation, then the added value of the result of a near patient test is limited, especially given the limited sensitivity of such tests.19 A probability of 50 per cent for accurate diagnosis is a reasonable basis for treatment, provided the prescribing decision is linked to that critical judgement.
In the retrospective analysis of symptom score based on patients consulting within 48 hours, a score exceeding 10 almost doubled the likelihood of confirmation compared with a score less than 10, something which perhaps could be exploited further in circumstances in which antiviral prescriptions are to be administered by pharmacists. However, the analysis of symptom severity scores did not discriminate between true influenza and other similar respiratory infections. There was a suggestion that pharmacists acquired a skill in recognising true influenza. The overall assessment of patients and their problems includes the demeanour and appearance of the patient and the non-verbal elements of communication. While we must encourage objective measurements, perhaps we underestimate the importance of these factors in the assessment process.
The study concerned patients presenting to pharmacists and that is not necessarily directly comparable with studies based on consultation in general medical practice. The study was restricted to patients who were at least well enough to visit a pharmacist and so excluded those experiencing particularly severe illness. Since access to pharmacists is not controlled by an appointment system, we might have expected relatively earlier presentation, although this did not happen. Patients were not subjected to any examination or investigation beyond having their temperature taken but even that did not demonstrate any added diagnostic benefit. The extra facilities at pharmacies needed for this study would not be required in a serious epidemic situation, where swab taking would not be routine.
Limitations of the study
The target number of patients was not recruited. The incidence of clinical illness in the population presenting to general practitioners was low in both years. In the first year this led to some delay in triggering the onset of the influenza active period and getting the study started. The predominant virus strain type was H3N2 in both years. Although it is not appropriate to analyse these data separately by year because of the small size of the study, we did not recognise any appreciable difference between the two seasons. As with all studies of influenza, the results relate to experiences in particular winters but the results are only likely to be better in winters in which influenza is more severe.
Recruitment to the study was biased, perhaps reflecting the difficulties pharmacists have in engaging in this sort of research and perhaps also a poor motivation towards research activity. Seven pharmacists did not recruit any patients and several recruited only one or two patients. One of the pharmacies recruited 73 per cent of all patients, potentially limiting the generalisability of the results, although the diagnostic accuracy of this pharmacist was similar to that of the other pharmacists. The high-recruiting pharmacy is located in a busy part of town, directly opposite a large health centre. The pharmacist has been established there for many years and is well acquainted with many of the patients, a situation typical of a busy community pharmacy in the UK.
Combined nose and throat swabs were used for PCR viral tests which are increasingly used routinely in general medical practice-based influenza surveillance in Europe.20 We did not assess the accuracy of pharmacist swab technique. Negative results do not necessarily mean that the patient does not have influenza; the test is most accurate for patients consulting within 48 hours of symptom onset, when viral replication is greatest in the period 24–72 hours after illness onset.9
Implications
The findings of the study have implications for routine pharmaceutical practice during a serious epidemic or pandemic situation. In a serious epidemic a higher positivity based on patient assessment might be expected, but it would be important for pharmacists to give advice in accordance with their judgement if antivirals are not to be used wastefully. Neuraminidase inhibitors can be purchased direct from pharmacists in some countries, but the potential for the development of drug resistance because of excessive and inappropriate use, needs to be considered carefully before increasing outlets for these drugs.
Prescribing supervision and strict limitation of prescribing periods would be needed. These limitations present a dilemma for pharmacists, since their business is to sell medicines rather than provide clinical advice which might often be “not to use them”, nor are they protected from the consequences of giving inappropriate advice. A practical resolution of these problems is needed if pharmacists are to have an extended role in influenza management. The findings of this study support increased involvement of community pharmacists in the clinical management of influenza epidemics.
Funding
The study was funded by Roche Products Ltd.
This paper was accepted for publication on 7 August 2007.
About the author
D. M. Fleming, PhD, FRCGP, is director of the Birmingham Research Unit of the Royal College of General Practitioners, Birmingham B17 9DB (e-mail dfleming@rcgpbhamresunit.nhs.uk)
References
- Ross AM, Kai J, Salter R, Ross J, Fleming DM. Presentation with influenza-like illness in generalpractice: implications for use of neuraminidase inhibitors. Communicable Disease and PublicHealth 2000;3:256–60.
- Department of Health. Choosing health through pharmacy: a programme for pharmaceuticalpublic health 2005–2015. London: Department of Health; 2005.
- Department of Health. A vision for pharmacy in the new NHS. London: Department of Health;2003.
- Blenkinsopp A, Noyce PR. Minor illness management in primary care: a review of communitypharmacy NHS schemes. Department of Medicines Management, Keele University; 2002.
- Hassell K, Whittington Z, Cantrill J, Bates F, Rogers A, Noyce P. Managing demand: transfer of management of self limiting conditions from general practice to community pharmacies. BMJ2001;323:146–7.
- Vohra S. A community pharmacy minor ailment scheme — effective, rapid and convenient.Pharmaceutical Journal 2006;276:754–6.
- Nicholson KG, Aoki FY, Osterhaus ADME, Trottier S, Carewicz O, Mercier CH, et al, on behalf ofthe Neuraminidase Inhibitor Flu Treatment Investigator Group. Efficacy and safety of oseltamivirin treatment of acute influenza: a randomised controlled trial. Lancet 2000;355:1845–50.
- Hayden FG, Osterhaus ADME, Treanor JJ, Fleming DM, Aoki FY, Nicholson KG, et al. Efficacy andsafety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections.New England Journal of Medicine. 1997;337:874–80.
- Moscona A. Neuraminidase inhibitors for influenza. New England Journal of Medicine 2005;353:1363–73.
- National Institute for Clinical Excellence. Guidance on the use of zanamivir, oseltamivir andamantadine for the treatment of influenza. Technology Appraisal Guidance No. 58; 2003.
- Ellis JS, Fleming DM, Zambon MC. Multiplex RT-PCR for surveillance of influenza in England in 1995/96. Journal of Clinical Microbiology 1998;35:2076–82.
- Snacken R, Influenza Diagnosis Working Party. Managing influenza in primary care. Disease Management and Health Outcomes 2000;8:79–85.
- Ehlers M, Silagy C, Fleming D, Freeman D. New approaches for managing influenza in primary care. Clinical Drug Investigation 2001;21:443–52.
- Fleming DM, Elliot AJ, Cross KW. Morbidity profiles of patients consulting during influenza and respiratory syncytial virus active periods. Epidemiology and Infection 2007;135:1099–108.
- Elliot AJ, Fleming DM. Surveillance of influenza-like illness in England and Wales during 1966–2006. Euro surveillance 2006;11; issue 10.
- Department of Health. Pandemic contingency plan. Available at: www.dh.gov.uk/pandemicflu/pandemiccontingencyplan/fs/en (accessed 31 August 2007).
- Aoki FY, Macleod MD, Paggiaro P, Carewicz O, El Sawy A, Wat C, et al, on behalf of IMPACT Study Group. Early administration of oral oseltamivir increases the benefits of influenza treatment. Journal of Antimicrobial Chemotherapy 2003;51:123–9.
- Hind C, Downie G. Vaccine administration in pharmacies — a Scottish success story. Pharmaceutical Journal 2006;277:134–6.
- Covalciuc KA, Webb KH, Carlson CA. Comparison of four clinical specimen types for detection of influenza A and B viruses by optical immunoassay and cell culture methods. Journal of Clinical Microbiology 1999;37:3971–4.
- Meijer A, Paget WJ, Meerhoff TJ, Brown CA, Meuwissen LE, Van der Velden J, on behalf of the European Influenza Surveillance Scheme. Epidemiological and virological assessment of influenza activity in Europe, during the 2004–2005 winter. Eurosurveillance 2006;11:111–8.


