Key points:
- National Institute for Health and Care Excellence (NICE) guideline CG180 for the management of atrial fibrillation (AF) recommends review of patients anticoagulated with suboptimal control on their current vitamin K antagonists (VKA, including if time in therapeutic range [TTR] <65%).
- The anticoagulant review clinic (ARC) was developed primarily to target this group of at-risk patients, supplementing work by GPs and utilising existing resources of the anticoagulant monitoring service (AMS).
- The AMS highlighted 1,431 patients to GPs with TTR <65% over 34 months; up to 60% were not reviewed and up to 80% remained on VKA.
- Implementation of the ARC increased the number of anticoagulant reviews by an estimated 34%. The number of patients remaining on VKA therapy (following GP review) decreased by an estimated 57%, shifting in favour of requesting ARC review.
- Under the ARC, 75% of patients were converted from their current VKA to a direct oral anticoagulant (DOAC). All three available DOACs were utilised. Less than a quarter of patients were prescribed reduced dose DOACs.
- Anonymous satisfaction survey overwhelmingly demonstrated the positive value of the service to the patients, including quality of information provided and joint decision making.
- Utilising a model of AF stroke-associated acute and post-acute healthcare costs averaging £17,293 per patient, the ARC is cost effective if it prevents only two strokes per year. This does not include cost savings from any reduction in major bleeds also achieved.
Introduction
Situation prior to intervention
The advent of direct oral anticoagulants (DOACs) has provided alternative oral anticoagulation options for the prevention of stroke and systemic embolism (SSE) secondary to non-valvular atrial fibrillation (NVAF). Historically, warfarin (and other vitamin K antagonists [VKAs]) had been the only suitable choice.
When the first DOAC (dabigatran) was licensed, the south east Essex AF committee (UK), comprised local commissioners and secondary care experts, devised and disseminated guidance for local GPs in December 2010 to manage patient selection for, and initiate treatment with, dabigatran. As a part of this initiative, the anticoagulation monitoring service (AMS) used an automated process to send a letter (termed a “low-time in therapeutic range [TTR] letter”) to patients’ GPs to highlight those patients currently receiving inadequate anticoagulation with a VKA for stroke prevention in AF. For this purpose, inadequate control was defined as those patients with:
- A total time with their international normalised ratio (INR) within the therapeutic range (TTR), using the INRs from the past six months, of less than 65%[1]
,[2]
, as calculated using the Rosendaal method[3]
, and; - An excess of ten INRs taken over the past six months. This was to exclude patients with marginally low TTRs skewed by long intervals (e.g. 12 weeks) with one or few exceptional low INRs, recognising a limitation of the linear interpolation used in the Rosendaal TTR calculation.
An internal audit of this process (from December 2010 to September 2013) identified that a minimum of 19.9% of patients for whom a low-TTR letter was sent were subsequently converted to a DOAC. Up to 59.3% received no follow-up from their GP, and of those patients who were reviewed, more than half (51.1%) were not converted to a DOAC, indicating limited uptake of DOAC prescribing in general practice. During this period, two further DOACs (apixaban and rivaroxaban) were granted marketing authorisation and all three received a positive National Institute for Health and Care Excellence (NICE) technology appraisal as an option for anticoagulation in NVAF[4]
,[5]
,[6]
. Each drug has unique characteristics, including dosing regimens, drug interactions and cautions for use[7]
,[8]
,[9]
, thus increasing the knowledge burden for GPs prescribing high-risk medicines[10]
,[11]
, with no head-to-head comparison to guide selection.
Establishing priorities
The 2010–2013 audit highlighted that up to 80% of this high-risk sub-group of NVAF patients (i.e. those that are inadequately anticoagulated) may not be receiving appropriate review and optimisation of their anticoagulant therapy.
Setting objectives: strategy
The audit results led to the development of the pharmacist-led ARC, which aimed to:
- Provide a review service for NVAF patients anticoagulated with a VKA to optimise their therapy and enable access to new NICE-approved technologies (DOACs) where appropriate;
- Continue to actively target the high-risk cohort of patients (NVAF on VKA with TTR<65%) for review using the low-TTR letters;
- Utilise existing infrastructure and specialist anticoagulation pharmacists from the centralised local anticoagulation monitoring service;
- Provide dedicated consultation for patients regarding their anticoagulation with an expert pharmacist;
- Enable joint, informed decision-making with the patient to optimise their anticoagulant therapy based on patient history; stroke/haemorrhagic risk; biochemical parameters; patient priorities and choice; drug-specific information (e.g. summary of product characteristics [SPCs]); and recommendations from local, national and international guidance;
- Ensure provision of the first months’ supply of the chosen anticoagulant with timely management of the switching process between anticoagulants, as necessary, to avoid discontinuity in care;
- Ensure provision of a management plan to referring practitioners including patient assessment details, (e.g. stroke/bleed risk scoring to ensure compliance with the Quality and Outcomes Framework [QOF])[12]
, ESC[13]
and NICE AF guidance[2]
), decision-making information, explaining the rationale for choice of anticoagulant (serving as an educational source) and ongoing monitoring requirements; - Follow up with each patient regarding their treatment plan to address any issues (e.g. potential adverse drug reactions) and provide advice or repeat appointments as necessary.
Mobilising resources
A business case was devised to establish the clinic, highlighting resource requirements to ensure suitable staff training, risk mitigation tools (e.g. addition of software modules to existing anticoagulation software), point-of-care testing equipment, clinic space and assistance, and staff time. Accordingly, the project was agreed locally as a pilot phase for six months, with all funding for resources met (and shared equally) by medical education grants from three pharmaceutical companies.
Method
The referral process to the anticoagulant review clinic (ARC) is outlined in ‘Box 1: Referral and review process for the anticoagulant review clinic (ARC)’. Referrals were permitted from physicians only and, as such, direct referral from the anticoagulation monitoring service to the ARC was not possible. The low-TTR letters were used to facilitate the referral process for the targeted high-risk cohort (NVAF on VKA with TTR<65% and >10 recalls for INRs over six months) by adding a simple reply-to-refer option for receiving physicians. Direct referrals were also accepted for patients established on VKA anticoagulation for NVAF, recognising that there may be scenarios other than a low TTR that warrant consideration of alternative therapy (e.g. needlephobia, patient preference and difficulty attending INR appointments). Referrals were rejected for patients not established on VKA treatment, or any patient with target INR >3 (e.g. range 3–4), mechanical heart valve in situ or indication(s) for anticoagulation other than NVAF. The ARC was a weekly, four-hour clinic run by an anticoagulation specialist pharmacist, which patients attended for six months. A comprehensive training package and standard operating procedure (SOP) was designed to support each practitioner, with a competence assessment carried out by the lead pharmacist and a consultant haematologist. A patient assessment proforma, approved by the haematology team, was embedded in the SOP for use in each consultation to ensure a consistent, high-quality assessment. Risk mitigation tools, including prescribing decision aids (either software or paper-based), were used to facilitate medication choice and supply. Since not all practitioners are registered prescribers, patient-group directions (PGDs) were developed to enable supply of all oral anticoagulants from the ARC. Building on existing links between the AMS and haematologists, further links with additional experts (e.g. consultant cardiologists and stroke physicians) were created to provide additional advice for complex patients (e.g. those with extensive cardiac history). Continuous audit of the ARC was to be conducted throughout the pilot phase, which ran from July 2014 to January 2015, measuring performance against standards, as well as collecting data for clinical analysis. Patient satisfaction was also measured via an anonymised survey.
Box 1: Referral and review process for the anticoagulant review clinic (ARC)
- Anticoagulation monitoring service identifies NVAF patients on VKA with TTR <65% (weekly).
- Low-TTR letter generated for each patient and sent to GP/responsible physician.
- GP/responsible physician refers the patient to the ARC by either:
- Replying to the low-TTR letter by ticking the option to refer to ARC (alternative is for physician to conduct review themselves), or
- Referring by letter direct to ARC (e.g. patient requiring review for issues other than low TTR).
- Patient contacted by ARC to have blood tests and permission to access medical records. Appointment given for ARC review.
- At ARC review: treatment decision made using full history, blood results and patient preference. Verbal and written information provided regarding treatment decision. A two-week telephone follow-up booked.
- Following ARC review, clinic letter generated and sent to GP (or other referrer) detailing outcome; copied to patient.
- Follow-up conducted; summary sent by letter to GP and patient. Patient discharged.
The performance standards for the ARC were primarily developed using:
- NICE guidance CG180[2]
(e.g. the choice and use of stroke and bleeding risk assessment tools); - The SPC for each DOAC[7]
,[8]
,[9]
and their internally developed PGDs (e.g. the need & mechanisms for assessing blood counts, liver and renal function); - Agreements with the local AF committee (e.g. clear, educational clinic letters to the referring physician).
A standard was also included to ensure a clear assessment of adherence. As mentioned in the NICE guidance[2]
, several factors may contribute towards unsatisfactory control of anticoagulation with a VKA (i.e. low TTR); the relative impact of many of these factors on successful anticoagulation is either diminished or eliminated by conversion to a DOAC, including drug-drug or drug-food interactions, interruption of therapy or metabolic phenotype (genetic variants of VKORC1 or CYP2C9 activity)[14]
,[15]
. A lack of anticoagulant control as a result of non-adherence is a complex issue unlikely to be solved by switching to an alternative treatment. Describing a clear adherence assessment and the causes of non-adherence in the design of the performance standards and the clinic assessment proforma ensured that each practitioner worked with the patient to find a solution. The solution may still include conversion to a DOAC, for example, in situations where the use of a medicine that is readily repackable in a monitored dosing system (MDS or ‘dosette box’) would improve adherence for that patient. The performance standards for the ARC required that:
- All patients reviewed have scores recorded for validated risk assessment tools (CHA2 DS2 -VASc and HAS-BLED) to inform treatment decisions;
- All patients reviewed have had assessment of INR, TTR, FBC, LFT and U&E/renal function prior to treatment decisions being made. In line with the SPC for each DOAC, renal function is calculated as creatinine clearance (CrCl) where possible using the Cockcroft-Gault equation[7]
,[8]
,[9]
; - All patients supplied with a DOAC by a pharmacist are within the marketing authorisation and PGD criteria for supply of that drug (where relevant);
- All patients consulted have had the risks and benefits of individual antithrombotics explained to enable informed decision making;
- All patients consulted have had an assessment of medication adherence and a discussion of barriers to this;
- All patients converted to an alternative antithrombotic will have received written literature relating to the medication in question, including an alert card;
- All patients converted to a DOAC will have been reviewed by telephone at two weeks to assess adherence, side effects and concerns, with appropriate action;
- All adverse reactions resulting from any change to anticoagulant therapy have been processed according to the Medicines and Healthcare products Regulatory Agency (MHRA) pharmacovigilance schemes (e.g. report all possible adverse reactions to “black triangle” drugs via the yellow card scheme);
- All patients reviewed under the service have had a letter detailing their appointment outcomes, including GP actions required, risk scores (where appropriate), history and assessment details, and explanation of the treatment decision made;
- All patients reviewed have not been reported/referred for inappropriate treatment decisions made under the service (measured throughout the audit period only).
The patient satisfaction survey standards required that all respondents:
- Were happy with the communication received prior to, and the reasons for attending, clinic review;
- Were happy with the level of efficiency on clinic day;
- Found staff friendly and polite;
- Found the information received from the pharmacist to be clear and concise;
- Had a better understanding of DOACs and the potential risks/benefits;
- Had the chance to ask questions unimpeded;
- Felt their opinions/concerns about anticoagulants were properly addressed;
- Did not feel pressured to make a particular decision;
- Were given clear contact information for further questions.
Results
The ARC six-month pilot phase ran from July 2014 to January 2015. During this period, 137 referrals were received, of which 87 patients were reviewed and subject to 100% achievement of all performance standards, with the exception of two patients (3.1%) who did not receive a two-week telephone follow up; in both cases alternative arrangements were made with the GP. One patient (1.15%) had a documented suspected adverse drug reaction (ADR), which was reported in accordance with the MHRA yellow card scheme.
Of the 87 patients reviewed, 70 patients (80%) submitted an anonymised patient satisfaction survey. In total, 100% of patients agreed with each of the survey questions, with the exception of one question relating to waiting times; 2 patients (3%) were not satisfied with the waiting time to be seen in clinic. Overall, 12 questions (1.7%) were unanswered from the returned surveys.
Table 1 describes the characteristics of the patients seen in the six-month pilot phase. The 87 patients were split almost equally between the two sexes with wide ranges observed for age, stroke risk score, renal function and TTR (with a mean of 54.2% but a range of 0–93%). Renal function was calculated as creatinine clearance using the Cockcroft-Gault equation[16]
in 82 of the 87 patients (94.3%), of which 13 (14.9%) results were adjusted on account of obesity. Estimated GFR (eGFR), using the modified diet in renal disease (MDRD) equation[16]
, was used for four patients (4.6%) in whom an accurate assessment of weight was unavailable. The assessment of renal function for the remaining one patient with obesity III was calculated by body surface area (BSA)-adjusted eGFR. Of the 87 patients, 65 patients (74.7%) were switched from their current VKA to a DOAC. One patient had their anticoagulation discontinued (haemorrhagic risk outweighed stroke risk), and no patients were converted to an alternative VKA. The remaining 21 patients (24.1%) continued their current VKA. Of those patients converted to a DOAC, all three available DOACs were utilised (see Table 2 for distribution) and less than a quarter (24.6%) were prescribed a DOAC at a reduced dose. Of those continuing on their current VKA, 16 patients (76.2%) declined a DOAC; the remaining were deemed to have contraindication(s)/relative caution(s) to conversion to a DOAC. Following conversion to a DOAC, 63 patients received two-week follow up, of which two patients (3.2%) discontinued their DOAC. Table 2 summarises the treatment outcomes of all 87 patients.
| Table 1: Demographical data of patients reviewed by the anticoagulant review clinic (ARC) | |
|---|---|
| Characteristics | n = 87 |
| Sex, n (%) | Male: 41 (47.1%) |
| Female: 46 (52.9%) | |
| Age, average years (range) | 76.9 (48-94) |
| CHADS2 average score (range) | 2.5 (0-5) |
| CHA2 DS2 VASc average score (range) | 4.4 (1-7) |
| HAS-BLED average score (range) | 2 (0-4) |
| CrCl*, n (%) | |
| >60 mL/min | 36 (41.4%) |
| 30-59 ml/min | 48 (55.2%) |
| <30 ml/min | 3 (3.4%) |
| Previous stroke/TIA, n (%) | 22 (25.3%) |
| Six-month TTR, % (range) | 54.2 (0-93) |
| *CrCl calculated using Cockcroft-Gault formula, adjusted in extremes of weight. MDRD formula used if weight unavailable. | |
| CrCl: creatinine clearance; MDRD: modified diet in renal disease; TIA: transient ishaemic attack; CHADS2 score: measure of stroke risk validated for patients with atrial fibrillation (AF), heart failure or left ventricular ejection = 1 point, diagnosed hypertension = 1 point, age >65 = 1 point, diabetes = 1 point, previous stroke or TIA = 2 points. CHA2 DS2 -VASc score = updated measure of stroke ris in AF patients, as for CHADS2 with the following changes: age 65-75 = 1 point, age >75 = 2 points, history of arterial disease (peripheral or cardiac, including previous myocardial infarction) = 1 point, female = 1 point. HAS-BLED score = bleeding risk tool validated for anticoagulated patients in AF; uncontrolled hypertension = 1 point, abnormal (severe) renal or hepatic impairment = 1 point (each), previous stroke = 1 point, labile INRs (TTR <60%) = 1 point, elderly (age >65) = 1 point, concomitant alcoholism = 1 point, and/or drugs known to increase bleeding risk (e.g. NSAIDs/antiplatelets) = 1 point. | |
| Table 2: Treatment outcomes of the anticoagulant review clinic (ARC) | |
|---|---|
| Number (%) of patients (total = 87) | |
| Change treatment | 66 (75.9%) |
| Direct oral anticoagulants (DOAC) | 65 (74.7%) |
| Apixaban | 42 (64.6%)* |
| Dabigatran | 8 (12.3%)* |
| Rivaroxaban | 15 (23.1%)* |
| Reduced dose DOAC | 16 (24.6%)* |
| Alternate vitamin K-antagonist | 0 (0%) |
| Stop anticoagulation | 1 (1.1%) |
| Continue current VKA | 21 (24.1%) |
| Not switched to DOAC because of: | |
| Compliance | 0 |
| Caution/contraindication | 5 (23.8%)** |
| Declined | 16 (76.2%)** |
| Two-week follow-up | 63 (96.9%) |
| DOAC stopped | 2 (3.1%) |
| *Percentage of all patients switched to any DOAC. **Percentage of all patients continuing on VKA. | |
The response to the low-TTR letters sent by the anticoagulation monitoring service was re-audited at the end of the six-month pilot phase. A total of 539 low-TTR letters were sent out, of which 295 (54.7%) responses were received, representing an increase of 34.4% in the number of responses seen from the initial audit. The majority of responses (n=137; 46.4%) requested the patient be reviewed in the ARC. A 57% reduction from the initial audit was noted in the percentage of responses from GPs, indicating the patient should continue their current VKA (n=80; 27.1% of all responses received).
The CONSORT diagram in Figure 1 illustrates the pathway from identification to ARC, including the patient numbers at each stage for the duration of the six-month study.
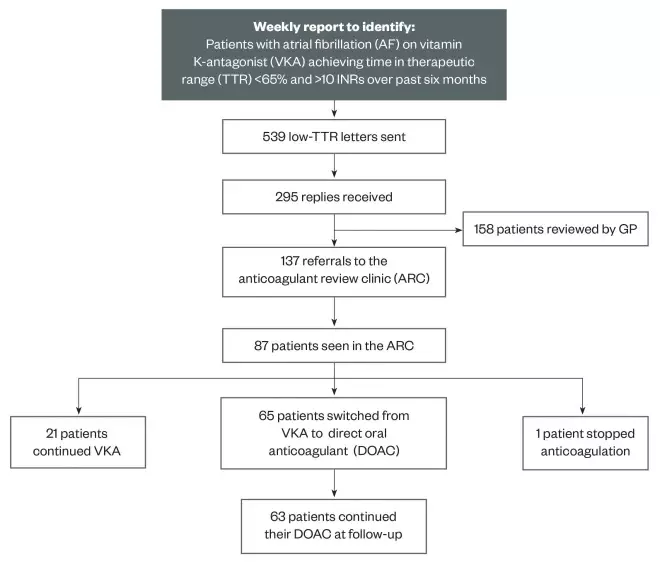
Figure 1: CONSORT diagram illustrating patient numbers from identification to review during the six-month ARC pilot
The CONSORT diagram illustrates the pathway from identification to the ARC, including the patient numbers at each stage, for the duration of the six-month pilot study.
Specific data describing the difference between the number of referrals received (n=137) and patients seen (n=87) were not collected, but did include reaching clinic capacity, patient non-attendance and incomplete or duplicate referrals. The planned clinic capacity was reduced following the observation that the predicted consultation time (30 minutes) fell short of the average measured consultation time (45 minutes).
Discussion and conclusions
The results show that the ARC met all the required performance standards, representing delivery of a consistently high quality service to all patients. Furthermore, the survey responses indicate that patients were highly satisfied with the service they received, with a minor exception with regards to waiting times.
The demographical data collected demonstrates that the average age (76.9 years) of patients consulted is higher than observed in the registration trials (64.5–71.6 years) for all three DOACs in NVAF available at the time[17]
,[18]
,[19]
, likely because of the area’s ageing population. The average CHADS2 score (2.5) is also higher than in two of the three registration trials (2.1)[17]
,[18]
,[19]
. In addition, more than half of the patients (58.6%) had a CrCl <60ml/min. Both observations may be attributable to the ageing demographic. History of stroke/transient ischaemic attack or myocardial infarction was similarly frequent in the ARC when compared with the registration trials[17]
,[18]
,[19]
. The average TTR of 54.2% is lower than observed in clinical trials, in line with the targeted patient group (TTR<65%), however, the extensive range should be noted (0-94%). Some patients reviewed by the ARC had TTR>65%; while these patients fall outside the high-risk group that were actively targeted, they met the criteria for ARC review (see ‘Process’) and each were referred for other treatment-related issues, such as needlephobia and collapsing veins, limiting adherence to obtaining INR results on a VKA.
In line with local and national guidance[2]
, Table 2 confirms all three DOACs were used by the ARC, with a notable skew towards apixaban (64.6% of patients switched to a DOAC). As identified by the demographical data, an ageing population with impaired renal function is likely to be responsible for this shift, on account of the favourable safety profile[20]
and limited renal clearance of apixaban [7]
,[8]
,[9]
,[21]
. In addition, in line with local guidance, ‘target dosing’ was used as an aid in DOAC choice, prompting the practitioner to use a DOAC at the upper licensed dose in preference to a DOAC at a reduced dose. This is owing to a paucity of data associated with reduced doses that, given the demographic, is likely to result in greater use of apixaban. Accordingly, less than a quarter of patients who switched (24.6%) received a reduced dose DOAC.
The initial low-TTR letter audit demonstrated that of those patients identified with TTR <65% on VKA therapy in whom GPs had reviewed, less than half (48.9%) were switched to a DOAC. By comparison, almost three-quarters (74.7%) seen by the ARC were converted to a DOAC, adding further evidence that, prior to the introduction of the ARC, at-risk patients were not receiving timely or sufficient review of anticoagulant therapy.
The two-week telephone follow-up demonstrated DOACs were well tolerated with a discontinuation rate of two patients (3.2%); one discontinued because of a possible allergic reaction and the second because of the risk of impaired absorption secondary to extensive colitis (apixaban-calibrated anti-Xa assay unavailable locally to confirm). Both patients were converted back to warfarin. While this discontinuation rate is lower than observed in clinical trial settings (20.7–25.3% over two years[17]
,[18]
,[19]
) and in a similar nurse-led service (22% over three months[22]
), it most likely represents a shortfall of the ARC service to the extent that continued follow-up was not offered during the project. The nurse-led service recorded that 37% of patients prescribed a DOAC required additional (unplanned) consultations with the nurse specialist[22]
.
Of the 21 patients not switched to a DOAC, patient preference towards their current VKA was the most common reason for not switching (16 patients; 76.2%), citing familiarity with their VKA, regular healthcare contact (via INR testing), reassurance of knowing the INR (as a measure of efficacy) and apprehension in using new medicines. Of the five patients not switched for clinical reasons, there were no absolute contraindications; two patients had CrCl <20ml/min, two required physician review for excessive bleeding risk, and one fell outside the studied population as their weight was over 150kg.
The assessment of adherence yielded several issues associated with sub-optimal anticoagulation with VKAs, for example potential drug-drug interactions, drug-illness interactions (including hospital admissions), and difficulty understanding and managing dose changes. The ageing demographic of the study population is a likely contributing factor to the high frequency of patients with multiple comorbidities, polypharmacy and recurrent decline in health (e.g. frequent infections) that can make management of VKA anticoagulation difficult. As discussed earlier, the relative impact of the majority of these factors is at least reduced by appropriate conversion to a DOAC. In many cases, however, no clear identifiable cause was found for sub-optimal anticoagulation. The assessment of adherence requires honest participation from the patient, which inevitably leads to a potentially unreliable conclusion, however, as discussed earlier, a patient’s metabolic phenotype (e.g. of VKORC1 or CYP2C9 activity) may also play a role in reducing the TTR in a manner not derivable from a medication adherence assessment[14]
,[15]
. In patients in whom poor adherence was identified, the cause may have been amenable to conversion to a DOAC. Not all cases can be analysed given the individual nature of the assessment and solution, however, some case types are discussed here. Where the cause was deemed to be a result of inability to fully understand or manage the dosing requirements of a VKA, and particularly if the rest of the patient’s regular medication is provided in MDS format, then a DOAC that could be added to an existing system was offered (e.g. apixaban or rivaroxaban). Where the patient’s routine meant that adherence to medication taken in the morning was high but at any other time of day was poor (a VKA is usually taken at 6pm), and said commitments also make availability for INR testing and clinical screening difficult, a once-daily DOAC was offered (e.g. rivaroxaban). Only two patients were deemed to have adherence issues that could not be solved within the capacity of the ARC, both of whom were already excluded from DOAC treatment for clinical reasons (they both required physician review for unacceptably high bleeding risk). For the 21 patients continuing on their VKA, strategies were suggested to improve adherence where this might have had an influence on their current TTR. In one case, the community pharmacist was involved to provide a prescription collection and delivery service for a patient who frequently ran out of warfarin, citing difficulty walking to the surgery and pharmacy to collect her prescription.
The ARC was designed to review patients already established on an anticoagulant, assuming that patients will already be familiar with much of the information being discussed in clinic. Despite this, an average consultation time of 45 minutes illustrates the continued need for input from healthcare practitioners in a patient’s ongoing treatment, with education aimed at supporting shared decision-making and positive patient outcomes.
Sustainability
With respect to patient numbers, the ARC is sustainable in the medium term. During the pilot period, 137 patient referrals were received and 87 patients were seen, distributed evenly throughout the six months. Patients with TTR <65% continue to be identified (539 patients throughout the six-month pilot phase). As the initial high-risk target population is addressed, referrals would be expected to decline. Accordingly, the function of the ARC could be expanded to include a number of similar roles, including (but not limited to) targeting and reviewing other high risk groups with AF (e.g. on antiplatelet only or no antithrombotic for stroke prevention), annual review for all anticoagulated patients, and reviewing anticoagulant choice and duration for VTE indications. Clinical resources are sustained as the ARC utilises existing specialists and infrastructure, limiting the chance of inability to provide the service. Additional financial input was required to run the ARC; in the current climate this makes such an initiative immediately unattractive to commissioners. The pilot phase was cost neutral, with all funding obtained via medical educational grants, however, this was only valid for six months. Although some costs were fixed initially (e.g. purchase of point-of-care testing), the majority of costs are for maintenance (e.g. staff time, clinic space allocation, consumables and software maintenance). A recent population analysis of stroke care in AF patients estimates the average acute cost of an AF-related stroke to be £10,413[23]
. Despite post-acute healthcare costs deemed to be non-significantly higher than pre-stroke healthcare costs, an additional average of £6,880 in long-term nursing/residential care was attributed directly to post-stroke care. Accordingly, commissioning the ARC long-term would need to prevent two additional strokes per year in the local population in order to be cost-effective. The authors recognise that for centralised anticoagulation services, capacity permitting, the ARC model may be integrated into existing care by re-engineering and optimisation of the VKA monitoring and dose adjustment processes, thus not requiring commissioning of any new services. The emerging availability of prescribing tools (e.g. the Keele University Prescribing Decision Support Tool[24]
) could have the potential to eliminate the need for expert anticoagulation practitioner input by guiding prescribers with respect to objective patient factors (e.g. age, weight and renal function). However, referring back to the average consultation time of 45 minutes per patient in the ARC, these tools do not replace the need for dedicated time for patient education.
Transferability
The ARC utilised existing staff and infrastructure from the AMS. Medical support for the monitoring service was already provided by the haematologists. In the context of the ARC, additional support from other specialties was also enlisted and essential to ensuring that all patients, including complex cases, received a high quality, clinically appropriate service. Medical advice, when sought, was distal to the clinic (either by phone, email or MDT), suggesting on-site presence is not required, however, such links are likely to only to be successful in centralised services where working relationships already exist (e.g. hospitals). Although local guidance was used as part of the ARC, there is established availability of national guidance and tools to facilitate safe and effective implementation of anticoagulation review services at other centres. The authors conclude that the ARC model is directly replicable by any centralised anticoagulation service staffed with dedicated specialist pharmacists or nurses.
Terry Dowling is principal pharmacist, haemostasis & thrombosis, Guy’s & St. Thomas’ NHS Foundation Trust and was formerly anticoagulation & oncology/haematology specialist pharmacist, Southend University Hospital NHS Foundation Trust. Ameta Patel is anticoagulation specialist pharmacist, Southend University Hospital NHS Foundation Trust. Kevin Oakley was deputy haematology lab manager and anticoagulation service lead, Pathology First, Southend University Hospital NHS Foundation Trust (now retired). Martin Sheppard is lead biologics pharmacist, Southend University Hospital NHS Foundation Trust. Correspondence to:
terry.dowling@gstt.nhs.uk
Financial and conflicts of interest disclosure:
Medical educational grants of equal proportion were received to fund start up and maintenance costs of the ARC for the six-month pilot phase from Bayer, Boehringer Ingelheim and Pfizer. TD has received speaker fees from Pfizer and travel grants from Bayer, Boehringer Ingelheim & Pfizer. AP and MS have received travel grants from Pfizer. No other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript have been made. No writing assistance was utilised in the production of this manuscript.
Acknowledgements:
The authors would like to thank Dr Guyler (stroke physician) for continued medical support and enthusiasm throughout the project, and to the South East Essex AF Committee for supporting the initiative.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Connolly SJ, Pogue J, Eikelboom J et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008;118(20):2029-2037. doi: 10.1161/CIRCULATIONAHA.107.750000
[2] National Clinical Guidelines Centre for the National Institute of Health & Care Excellence. Atrial Fibrillation: the management of atrial fibrillation. (London: National Institute for Health & Care Excellence, 2014). Available at: http://www.nice.org.uk/cg180 (accessed June 2016).
[3] Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thrombosis and Haemostasis 1993;69(3):236-239. PMID: 8470047
[4] National Institute for Health and Care Excellence. Apixaban for preventing stroke and systemic embolism in people with nonvalvular atrial fibrillation (TA275). (London: National Institute for Health & Care Excellence, 2013). Available at: http://www.nice.org.uk/ta275 (accessed June 2016).
[5] National Institute for Health and Care Excellence. Dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation (TA249). (London: National Institute for Health & Care Excellence, 2012). Available at: http://www.nice.org.uk/ta249 (accessed June 2016).
[6] National Institute for Health and Care Excellence. Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation (TA256). (London: National Institute for Health & Care Excellence, 2012). Available at: http://www.nice.org.uk/ta256 (accessed June 2016).
[7] Boehringer Ingelheim Ltd. Pradaxa 150mg hard capsule: Summary of Product Characteristics. Available at: http://www.medicines.org.uk (accessed June 2016).
[8] Bristol-Myers Squibb – Pfizer Alliance. Eliquis 5mg film-coated tablet: Summary of Product Characteristics. Available at: http://www.medicines.org.uk (accessed 6th January 2016).
[9] Bayer plc. Xarelto 20mg film coated tablet: Summary of Product Characteristics. Available at: http://www.medicines.org.uk (accessed June 2016).
[10] National Patient Safety Agency. Patient Safety Observatory Report 4. Safety in Doses. (London: National Patient Safety Agency, 2007). Available at: http://www.nrls.npsa.nhs.uk/resources/patient-safety-topics/medication-safety (accessed June 2016).
[11] National Patient Safety Agency. Patient Safety Alert 18: Actions that can make anticoagulant therapy safer. (London: National Patient Safety Agency, 2007). Available at: http://www.nrls.npsa.nhs.uk/resources/patient-safety-topics/medication-safety (accessed June 2016).
[12] NHS England/NHS Employers. 2014/15 General Medical Services (GMS) Contract Quality and Outcomes Framework (QOF): Guidance for GMS Contract 2014/15. (London: NHS Confederation (Employers), 2014).
[13] Camm AJ, Lip GYH, De Caterina R et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. European Heart Journal 2012;33:2719-2747. doi: 10.1093/eurheartj/ehs253
[14] Reitsma PH, van der Heijden JF, Groot AP et al. A C1173T dimorphism in the VKORC1 gene determines coumarin sensitivity and bleeding risk. PLoS Medicine 2005;2:e312. doi: 10.1371/journal.pmed.0020312
[15] Higashi MK, Veenstra DL, Kondo LM et al. Association between CYP2C9 genetic variants and anticoagulation related outcomes during warfarin therapy. JAMA 2002;287:1690-1698. doi: 10.1001/jama.287.13.1690
[16] Botev R, Mallié J-P, Couchoud C et al. Estimating Glomerular Filtration Rate: Cockcroft–Gault and Modification of Diet in Renal Disease Formulas Compared to Renal Inulin Clearance. Clin J Am Soc Nephrol 2009;4(5):899-906. doi: 10.2215/CJN.05371008
[17] Granger CB, Alexander JH, McMurray JJV et al. Apixaban versus warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine 2011;365(11):981-992. doi: 10.1056/NEJMoa1107039
[18] Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine 2009;361(9):1139-1151. doi: 10.1056/NEJMoa0905561
[19] Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. New England Journal of Medicine 2011;365(10):883-891. doi: 10.1056/NEJMoa1009638
[20] Mitchell SA, Simon TA, Raza S et al. The Efficacy and Safety of Oral Anticoagulants in Warfarin-Suitable Patients with Nonvalvular Atrial Fibrillation: Systematic Review and Meta-Analysis. Clinical and Applied Thrombosis/Haemostasis 2013;19(6):619-631. doi: 10.1177/1076029613486539
[21] Heidbuchel H, Verhamme P, Alings M et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625-651. doi: 10.1093/europace/eut083
[22] Folkeringa RJ, Geven LM, Veldhuis T et al. Practical introduction of novel oral anticoagulants through an anticoagulation nurse. The Leeuwarden model. Netherlands Heart Journal 2014;22(6):297–300. doi: 10.1007/s12471-014-0529-9
[23] Luengo-Fernandez R, Yiin GSC, Gray AM, et al. Population-based study of acute- and long-term care costs after stroke in patients with AF. International Journal of Stroke 2013;8(5):308-314. doi: 10.1111/j.1747-4949.2012.00812.x
[24] Keele University Prescribing Decision Support, Centre for Medicines Optimisation anticoagulation therapy decision support tool. Available at: http://www.anticoagulation-dst.co.uk (accessed January 2016).
You may also be interested in
Pharmacists made 150,000 patient interventions during anticoagulant safety audits, report finds
Some patients switched to edoxaban without being properly informed, pharmacists say