Introduction
NHS bodies are legally required to have an antimicrobial prescribing policy in place.[1]
This policy is recommended to include antimicrobials that are restricted (ie, need authorisation), unrestricted or permitted for specific indications. Restricted antibiotics are those likely to contribute to the development of multi-resistant organisms or those that are more costly. They are generally broad-spectrum antibiotics, such as cefuroxime and ciprofloxacin, to which resistance emerges rapidly. Patients treated with these antibiotics are often at a greater risk of developing healthcare-associated infections (HCAIs), such as Clostridium difficile.[2]
Prudent use of all, and restriction of some, antimicrobials will reduce the risk of antimicrobial resistance and development of HCAIs.[2],[3],[4]
,[5]
Antimicrobial policies guiding prescribing should be reviewed regularly and compliance should be monitored.[3]
The Department of Health provides a framework for trusts to review their prescribing practices, to ensure safe and appropriate prescribing of antimicrobials.[3]
In 2007, Cooke and Holmes suggested that a specific antibiotic care bundle should be available.[6]
At that stage there were other high-impact interventions (HIIs) already available to tackle HCAIs, including: tackling microbial contamination; insertion and care of peripheral and central lines and urinary catheters; care of ventilated patients; prevention and management of Clostridium difficile diarrhoea; and prevention of surgical-site infection.[3] Since then, one more HII (cleaning and decontamination of medical equipment) has been introduced and implemented across NHS trusts. The care bundle for antimicrobial prescribing was published in draft form in March 2010.[7]
The issue
Various studies have shown that the clinical rationale for starting antimicrobials is poorly documented and this often makes the decision to review or stop treatment more difficult.[4],[5],[6],[7],[8],[9],[10]
In addition, medication charts in acute settings generally have 14 administration days per prescription. This often leads to extended administration of antimicrobials, particularly for patients receiving several medicines or those with untidy or unclear medication charts.
Mid Essex Hospital Services NHS Trust includes a 700-bed district general hospital and a specialist tertiary referral centre, St Andrew’s Centre for Plastic Surgery and Burns, which also provides head and neck surgical services. Antimicrobial prescribing within the trust is regularly monitored via six-monthly point prevalence and targeted audits. In addition, monthly audits on antimicrobial prescribing are carried out for the trust’s “Infection control score card”, part of the monthly director of infection prevention and control report to the primary care trust. The monthly audit involves one ward from each of the five divisions within the trust — emergency care and medicine; theatres and anaesthetics; planned care; specialist surgery; women and children — being audited for antimicrobial prescribing by a clinical pharmacist or technician during their normal clinical ward visit. Data are collected using the one-day point prevalence method on a rolling basis and include patient name, antimicrobial dose, frequency and indication. However, the monthly audits focus on documentation of the clinical rationale for prescribing and evidence of review (in the medical notes or on the drug chart) within five days of starting therapy.
The standard set by the PCT was that 85% of all antibiotic prescriptions must have the clinical indication documented and evidence of review within five days (ascertained by either the duration of the course or review comments documented in the medical records). These two criteria are based on recommendations from the DH’s “Saving lives: antimicrobial prescribing: a summary of best practice”.[3] The 85% standard was set as a consensus between the trust board and PCT commissioners, who request that these data are available on the trust’s “Infection control score card”.
In the first three years of regular monthly audits, the 85% standard was not achieved. Yearly trust-wide one-day point prevalence antimicrobial audits (n = 192 [2007]; 165 [2008]; 252 [2009]) revealed that less than 60% of antimicrobial prescriptions audited had the clinical indication documented in the medical records. Evidence of review within five days was also poor at less than 40%.[8]
Strategies for improvement
Over the years, several initiatives were attempted to improve antimicrobial prescribing, including regular feedback to prescribers, performance management of wards with poor antimicrobial prescribing, the introduction of pocket-sized antimicrobial cards, pharmacy interventions (such as drawing a box around the space for administration on the drug chart on day 7), documentation in notes and use of stickers on the drug chart to suggest and encourage review. Despite these initiatives, audit results remained poor.
During 2009 the redesign of the trust’s drug chart was used as an opportunity to include a dedicated section for antimicrobial prescribing (see Figure 1). The antimicrobial section on the new chart has a prompt and space for documenting the indication for each drug prescribed. Also, for each prescription the number of days of administration is limited to five, after which the prescription must be reviewed and prescribed again, if appropriate.
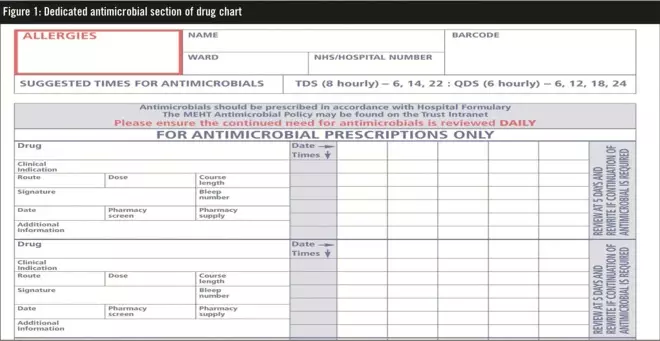
Figure 1. Dedicated antimicrobial section of drug chart
A separate section on the front of the chart was introduced for prescribing and administering antimicrobial surgical prophylaxis (see Figure 2). Several other sections were also introduced on the new drug chart including oxygen prescribing, a pre-printed section for venous thromboembolism prophylaxis and pharmacy medicines reconciliation. The allergy section was also revised so that it now includes allergies, sensitivities and a prompt for documenting the type and nature of any reactions (Figure 2).
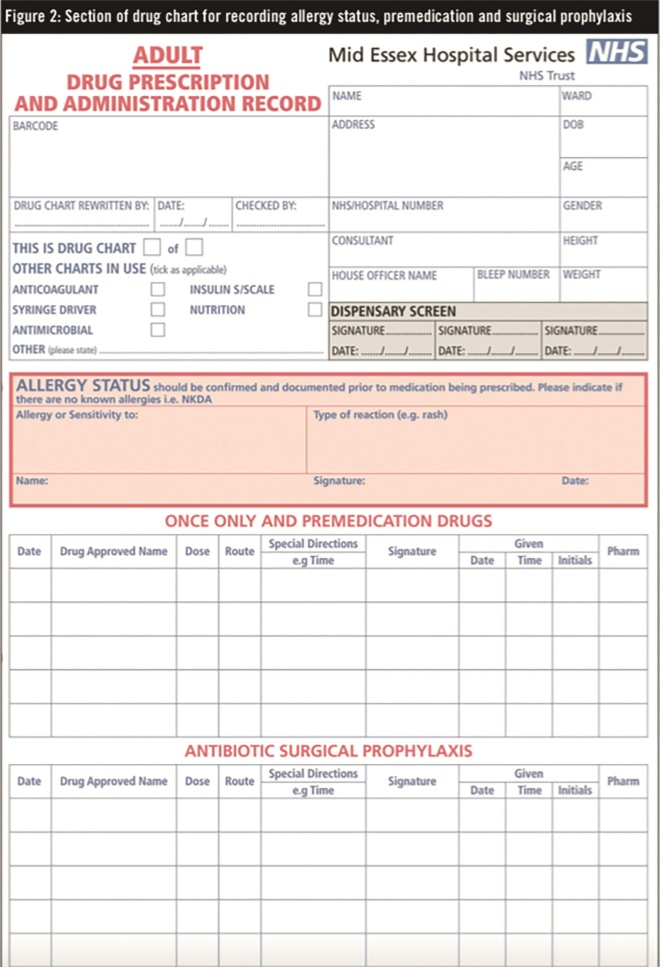
Figure 2. Section of drug chart for recording allergy status, premedication and surgical prophylaxis
Aim
The aim of this investigation was to assess the impact of the new drug chart on antimicrobial prescribing, with specific regard to the trust-wide standard that 85% of patients must have the antimicrobial indication documented and evidence of review within five days.
Method
Data collected during the trust’s regular audit and monitoring of antimicrobials included patient demographics (hospital number, initials and date of birth — these were anonymised during data collation but were required to allow for any necessary clinical follow-up), antimicrobial prescribed, dose, route, frequency of administration, clinical indication, duration (if documented), sources of documentation (drug chart or notes) and evidence of microbiology approval recorded in the notes for restricted antimicrobials. All antimicrobials and routes of administration were included in the data collection.
This report presents an analysis of the data obtained from the antimicrobial audits in the six-month periods before and after the implementation of a dedicated antimicrobial section on the adult medication chart (January to June in 2009 and 2010) with respect to indication documentation and review of antimicrobials.
Point-prevalence monthly data for all prescribed antimicrobials were collected by clinical pharmacists during their usual ward visits on the allocated month. For the monthly audit, one ward per division is audited on a rolling basis. There were changes to the ward configuration within the trust over the two years but the divisions included in the study and the numbers of wards per division were similar in both periods.
Since this was an audit of practice against agreed standards, ethics approval was not required. The audit was registered with the trust’s clinical audit department and followed the clinical audit guidelines of Mid Essex Hospital Services NHS Trust.
Exclusion criteria
Patients in day-stay surgical wards and those visiting outpatient clinics were excluded from the audit because the trust antimicrobial policy applies mainly to inpatients, who have an increased risk of developing HCAIs. Anaesthetics and theatres were also excluded from this study since only prophylactic antibiotics are administered in these areas.
Results
The results show that the introduction of the new drug chart with a dedicated antimicrobial prescribing section improved the documentation of the indication for prescribed antimicrobials and also review within five days. In the pre-implementation audit 207 antimicrobial prescriptions were reviewed; following implementation, 165 antimicrobial prescriptions were audited. In the six months before the new drug chart was introduced, audit data showed that less than a third of antimicrobial prescriptions (n=69) were reviewed within five days and the indication was documented in 63% of cases (n=131). Following the introduction of the drug chart, documentation of indication increased to 89% (n=147) and review within five days to 84% (n=138) — see Box 1. Although the absolute increase of 26% for clinical indication documentation was not statistically significant, the 50%improvement in review of antimicrobials within five days was statistically significant (P<0.005).
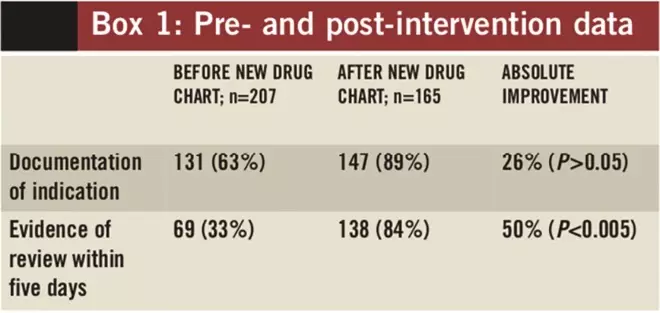
Box 1. Pre- and post-intervention data
Discussion
The results show that introduction of the new drug chart improved documentation of indication and review of therapy by 26% and 50%, respectively and, crucially, the 85% standard for documentation of clinical indication was met. The results (particularly evidence of review) are expected to improve further with continued familiarity with the new drug chart and feedback of the monthly audit results to medical, nursing and pharmacy staff, as well as to divisional clinical managers (who are now performance managed on ensuring that their teams meet the antimicrobial prescribing criteria audited).
Although issues around confidentiality were raised initially with regard to documenting the indication of antimicrobials on drug charts, this has not been highlighted as a problem in practice following introduction of the new drug chart.
A formal training programme on antimicrobial prescribing has been included in the infection prevention and control training competency (passport) for foundation year 1 and 2 doctors. Since August 2010, these doctors are given a 60 question quiz on antimicrobial prescribing and stewardship to complete. They are individually assessed as competent and this now forms a mandatory component of the foundation portfolio. Directorate audit days and business meetings are used for feedback and educational opportunities for more senior medical staff, registrars and consultants.
In addition to improving antimicrobial prescribing, the new drug chart has also been observed to improve the screening of antimicrobial prescriptions for appropriateness by pharmacists, especially when medical notes are not available.
A potential limitation of this study is that there was no control group. It is possible that improvements in awareness or education of prescribers about principles of antimicrobial prescribing led to changes in prescribing behaviour unrelated to the drug chart intervention. However, this is thought to be unlikely because, until August 2010, education and training on the general principles of antimicrobial prescribing had been taking place for at least two years.
Other methods have been used in an attempt to improve antimicrobial prescribing including writing in notes, and use of stickers and other manual reminders. However, data from previous point prevalence audits (2007–09) showed that none of these interventions had been effective and they are time consuming. Having a drug chart that includes a section for documenting clinical indication and that also limits the number of administration days, ensures adherence to DH recommendations on prudent antimicrobial prescribing without extra effort from the prescribers. Cooke and colleagues have suggested that the “introduction of user- friendly practical systems to prompt, reinforce and aid practice through facilitating simple documentation of indications for treatment and review dates should help optimise behaviours and ultimately, patient care”.[10]
We show from this audit that having a dedicated antimicrobial prescribing section within prescription charts is one such system that can be effective.
Conclusion
Including an antimicrobial prescribing section as part of the hospital’s prescription chart meant that the trust’s 85%target for documenting the indication for antimicrobials was met and that the number of antimicrobial prescriptions that were reviewed within five days improved significantly. Absolute improvements of 26% and 50% were observed for the documentation of indication and review within five days, respectively.
Further educational sessions and an antimicrobial prescribing competence programme for junior doctors have also been introduced to the trust to ensure sustained improvements in antimicrobial stewardship. The results of the audit show that a dedicated section on the drug chart for antimicrobial prescribing is a user-friendly, practical system to help improve antimicrobial stewardship.
About the authors
Diane Ashiru-Oredope was, at the time of writing, lead clinical pharmacist for antimicrobials, Maria Richards is lead clinical pharmacist for admissions, Jane Giles is chief pharmacist, Nicola Smith is lead clinical pharmacist for antimicrobials and Louise Teare is consultant microbiologist and director of infection prevention and control, all at Mid Essex Hospital Services NHS Trust.
Email: diane.ashiru-oredope@nhs.net
References
[1] Department of Health. The Health and Social Care Act 2008: code of practice for the NHS on the prevention and control of healthcare associated infections and related guidance. January 2009. www.dh.gov.uk/en/Publicationsandstatistics/ Publications/PublicationsPolicyAndGuidance/ DH_093762 (accessed 20 December 2009).
[2] Gould IM. Antimicrobial policies to control hospital-acquired infection. Journal of Antimicrobial Chemotherapy 2008;61:763–5.
[3] Department of Health. Saving Lives: reducing infection, delivering clean and safe care — Antimicrobial prescribing, a summary of best practice. 2007. www.dh.gov.uk/en/ Publicationsandstatistics/Publications/ PublicationsPolicyAndGuidance/DH_078134 (accessed 31 August 2010).
[4] Wickens HJ, Jacklin A. Impact of the hospital pharmacy initiative for promoting prudent use of antimicrobials in hospitals in England. Journal of Antimicrobial Chemotherapy 2006;58:1230–7.
[5] Davey P, Brown E, Fenelon L, et al. Interventions to improve antimicrobial prescribing practices for hospital inpatients. Cochrane Database of Systematic Reviews 2005;issue 4.
[6] Cooke FJ, Holmes AH. The missing care bundle: antimicrobial prescribing in hospitals. International Journal of Antimicrobial Agents 2007;30:25–9.
[7] Department of Health. High impact intervention — antimicrobial prescribing care bundle. 2010. http://hcai.dh.gov.uk/whatdoido/high-impact-interventions (accessed 31 August 2010).
[8] Ashiru DAI, Benton J, Lee R, et al. Antimicrobial usage over three years at a district general hospital. Journal of Pharmacoepidemiology and Drug Safety 2010;19:645–9.
[9] Gnanarajah DP, Jones D, Cheesbrough J. Can “antimicrobial ward rounds” improve prescribing? Journal of infection 2007;55:e81.
[10] Cooke FJ, Matar R, Lawson W, et al. Comment on: Antimicrobial stewardship — more education and regulation not more availability? Journal of Antimicrobial Chemotherapy 2010;65(3):598.