Abstract
Aim
To evaluate clinical interventions in cancer services made by pharmacists on the wards and in the dispensing of chemotherapy doses to assess pharmacists’ contribution to patient care.
Design
A prospective cross-sectional survey.
Subjects and setting
Nine clinical pharmacists providing services on six cancer wards and at the chemotherapy production unit (CPU) at Barts and The London NHS Trust, London.
Outcome measures
The type and frequency of clinical interventions made by pharmacists and their significance to patient care, as rated by an expert panel of pharmacy staff.
Results
115 pharmacist interventions on 340 cases (prescriptions, chemotherapy doses or patients) were observed. In 94% of cases, the pharmacist’s advice was accepted by medical practitioners. The panel rated 64% of interventions to have a significant, very significant or potentially life-saving effect on patient care. The panel and a consultant medical oncologist reached agreement on the significance rating of only 17% of a sample of interventions; however, both rated 77% of the interventions to be significant, very significant or potentially life-saving. At the CPU, most interventions involved cancer therapy and were considered more significant than those made on the wards which mainly involved support therapy. Pharmacists of higher grades contributed more to care of patients on complex drug therapy, whose therapy required more significant interventions.
Conclusions
Interventions made by pharmacists are related to the level of practice, with more complex interventions made by more senior or experienced staff. Pharmacist interventions can improve the standard of patient care, reduce risk and prevent major toxicity. The contribution of pharmacists is required for high quality services.
The management of cost, access and safety of medicines have become central roles for pharmacy. Over the past decade, the NHS has been redesigned to offer services around the needs of patients, placing quality improvement at the top of the NHS agenda.1–3 Pharmacists are involved in the prescribing and supply of medicines, the provision of clinical services and interventions to improve the management of medicines.4–7 Interventions made by pharmacists have been shown to improve therapeutic benefit,8–14 anticipate medication errors6,14 and provide a better use of financial resources.12,15
Managing cancer care
Improvement of cancer services is one of the priorities in the NHS.1,3,16 With the development of new drugs and technologies, the management of certain cancers has evolved from that of an acute disease to a chronic illness. Each week 5,000 people in the UK are diagnosed with cancer.17 While technological advances in cancer therapies mean more treatments are available for more patients with more complex forms of the disease, the drugs are often highly toxic. Management of the risks and costs associated with cancer therapies requires the involvement of pharmacists in the production of chemotherapy and in the provision of advice to maximise patient safety.18,19 Advice given by pharmacists has been found to improve patients’ control over chemotherapy side effects.13 Pharmacists are able to minimise medication errors and have a central role in assuring patient safety.6,14,16 The contribution of pharmacists needs to be evaluated at each stage of the treatment of oncology patients to assure the quality of services.1,21 This study evaluated the clinical interventions made by pharmacists in cancer services at a tertiary care centre in London.
Methods
Setting and subjects
The study was conducted at St Bartholomew’s Hospital, a tertiary centre for cancer treatment for North East London and part of the Barts and The London NHS Trust. Interventions were recorded at the chemotherapy production unit (CPU) and on the wards. For the purpose of this study, the inpatient wards and outpatient day units were regarded as wards — at the time of the study there was no pharmacist permanently in charge at the day units. The specialties of the wards were medical, clinical oncology, haematological malignancy and transplants. Nine pharmacists worked in cancer services. They were graded B to E (approximately equivalent to AfC bands 6–8a).
Ethics approval was not required as the study was part of the trust’s service development. However, the study protocol was reviewed by two independent researchers at the School of Pharmacy, University of London, to assess any ethical issues.
Developing the data collection form
The study was a prospective cross-sectional survey of clinical interventions made by pharmacists in cancer services. To standardise the recording of interventions, a data collection form was developed, using the literature in the area,8–12, 22–27 observations of pharmacists at both the CPU and on the cancer wards, and discussions with pharmacists.
The data collection form had two parts: one section for recording information such as a pharmacist’s grade, the activity carried out, and the treatment and health condition of a patient, and one for describing the intervention, for example, the drugs involved, the identified problem, the intervention, contact with any other healthcare professional and whether the advice on an intervention was accepted. The problems identified by the pharmacists were classified as “clerical”, “drug and therapy”, “dose, frequency and duration”, “administration and formulation” and “storage and dispensing.”11,12, 22–27 The form and the data collection were piloted in the CPU and ward settings. Minor amendments were made to the form which was then used throughout the study.
Data collection
At the CPU, the clinical activities of pharmacists were observed during prescription screening, where the appropriateness and safety of prescribed treatment was confirmed, and during drug release, where the accuracy of the chemotherapy dose dispensing and the patient’s health condition was confirmed before sending the dispensed medicines to be administered to the patient. On the wards, pharmacists provided a wide range of clinical services but activities during the endorsement of drug charts and “to take away” (TTA) forms were particularly observed.
Data collection was planned to ensure a representative sample of the interventions made on the different wards and in the CPU at different times during week days. A wide range of observations was made to capture different levels of activity. The data were collected by two researchers who independently shadowed pharmacists during nine CPU visits and 25 ward visits. This allowed an objective measure of interventions in practice.22
Expert panel
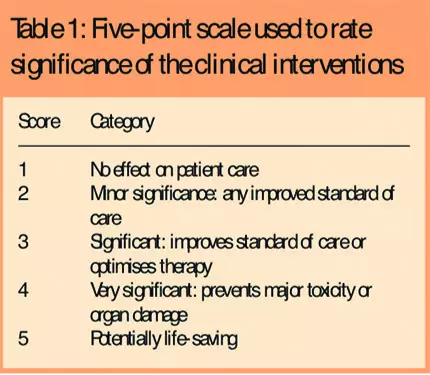
The clinical impact of the pharmacists’ interventions was assessed by an expert panel of pharmacy staff, comprising the head of production services, three clinical oncology pharmacists, and a specialist in drug manufacture and drug stability. This session was facilitated by three researchers. The panel was provided with a summary of each recorded intervention. Each intervention was discussed until the panel reached a consensus on its significance. The significance of the recorded interventions was rated on a fivepoint Likert scale, with categories ranging from 1 = “no effect” to 5 = “potentially life saving” (Table 1).
To validate the significance of the interventions further, a consultant in medical oncology rated a sample of the interventions. After correcting for chance, the agreement between the panel and the consultant (weighted κ for significance of the interventions) was 0.17 (n=56, P=0.031), indicating 17 per cent agreement. This level of agreement was surprisingly low. The classifications were reviewed a second time and it was found that in 91 per cent of interventions the panel and the consultant agreed or differed by only one category (higher or lower). The differences were therefore not as marked as first thought. Both the panel and the consultant rated 77 per cent of the interventions to have been “significant”, “very significant” or “potentially life saving”. In subsequent analyses, the rating of the panel was used.
Data handling and analysis
Confidentiality and anonymity of both patients and pharmacists was ensured throughout. The data were coded and entered on to an SPSS version 14 database and quality assured to ensure data coding and entry errors were minimal. Non-parametric tests, such as chi-squared, Mann-Whitney U and Kruskal-Wallis H, and parametric tests, such as ANOVA, were used to test for any associations and differences between variables.
Results
Setting and patients
Interventions were made to the prescriptions, the chemotherapy doses, the drug charts or the TTA forms of 71 patients. Sixty-six per cent (n=47/71) of the patients who required an intervention were female, with a median age of 53 (range 18 to 87). The majority were being treated for a haematological malignancy (47 per cent, n=33/70) or medical malignancy (43 per cent, n=30/70). The patients were prescribed a median of 10 drugs (range two to 16), which included a high number of known toxic drugs, and therefore represented an at-risk patient population. Patients were often in hospital for a scheduled chemotherapy appointment (52 per cent, n=35/68) or because of side effects caused by chemotherapy, such as sickness or infection (20 per cent, n=14/68). The latter was particularly common in elderly patients (ANOVA, F=3.824, P=0.008).
Pharmacists
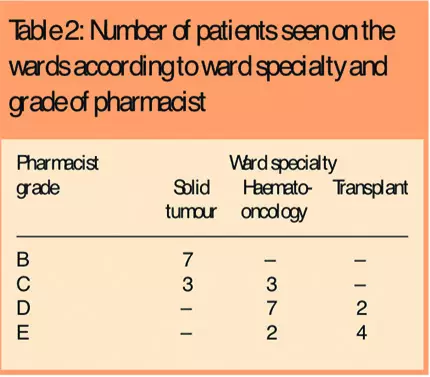
The nine pharmacists were allocated to the different wards according to their expertise (χ2=92.652, P<0.0005), and they were involved in managing the medicines of patients in different cancer specialties (χ2=28.078, P<0.0005). Lower-grade pharmacists (B and C) tended to be employed on wards where patients with solid tumours were treated, higher grade pharmacists (grades C, D and E) were based on wards treating haematological malignancy and more experienced pharmacists (grades D and E) were based on transplant wards (Table 2).
Interventions
Pharmacists were observed working on 340 cases (prescriptions, chemotherapy doses or patients). This represented 130 prescriptions screened and chemotherapy doses released at the CPU, and 210 patients on the wards. Overall, 115 interventions were made: 21 at the CPU and 94 on the wards and outpatient day units. These involved 76 cases, with some requiring more than one intervention: a mean of 1.5 interventions were made per case. However, at the two day units, where there was no permanent pharmacist service during the study, only one intervention was observed during six visits.
Clinical interventions were most commonly associated with “drug and therapy” problems (38 per cent, n=44/115), followed by “dose, frequency and duration” (32 per cent, n=37/115) and “clerical” problems (20 per cent, n=23/115) (Table 3).
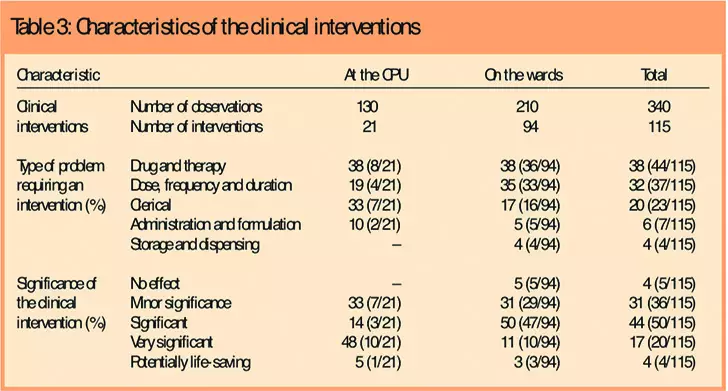
There were few clinical interventions related to “administration and formulation” (6 per cent, n=7/115) and “storage and dispensing” (4 per cent, n=4/115). Problems related to “drug and therapy” were common both at the CPU and on the wards (38 per cent, n=8/21; and 38 per cent, n=36/94), whereas “clerical” problems were common at the CPU (33 per cent, n=7/21) and “dose, frequency and duration” problems were frequent on the wards (35 per cent, n=33/94).
The impact of the clinical interventions made by pharmacists on patient care was rated to be “significant”, “very significant” or “potentially life saving” in the majority of cases (64 per cent, n=74/115). Medical practitioners accepted advice on most of the interventions proposed by pharmacists (94 per cent, n=108/115).
Exploring the interventions
There were differences between interventions made in the CPU and those made on the wards. Most interventions made at the CPU were related to the treatment of solid tumours, while pharmacists on the wards intervened on treatments for haematological malignancy (χ2=6.197, P=0.041).
Different types of drugs were involved in the interventions at the CPU and on the wards (χ2=80.656, P<0.0005); the interventions made at the CPU mostly involved cancer drugs (92 per cent, n=23/25),whereas interventions on the wards commonly concerned support therapy (71 per cent, n=70/98).
The interventions recorded at the CPU had a higher significance rating than those recorded on the wards: 48 per cent of interventions (n=10/21) at the CPU had a “very significant” impact on patient care compared with 50 per cent (n=47/94) rated as “significant” on the wards (Mann-Whitney U, z=2.116, P=0.033). On the wards, the treatment of transplant patients required more “significant” (or complex) interventions, followed by the treatment of patients with haematological malignancy and patients with solid tumours (Kruskal-Wallis H, χ2=12.131, P=0.002). Pharmacists of higher grades made more significant interventions on the wards (Kruskal-Wallis H, χ2=9.999, P=0.019),probably because the patients whose medicines they were managing require more complex treatment with higher levels of toxicity and associated risks.
Discussion
This study evaluated the clinical pharmacy services provided in the dispensing of chemotherapy doses and on the wards, by peer review of the impact of interventions made by pharmacists on patient care.
Strengths and limitations
The cross-sectional study was limited to nine CPU visits and 25 ward visits at one hospital. The results are not generalisable but the study sought to provide insight into pharmacy interventions in the management of medicines in cancer patients. The agreement between the panel and the consultant was low at 17 per cent. However, the significance of interventions was rated either the same or one category lower or higher in 91 per cent of the cases; this could indicate that the rating criteria were too broad. Perhaps more interesting is the need for different disciplines to work together, each bringing their expertise and experience to improve patient care to obtain a “true” indication of the significance of such interventions.
Pharmacists’ interventions
Cancer patients are often in poor health and the chemotherapy they receive can be aggressive.18 Patients requiring interventions to their therapies tended to have been prescribed many drugs and had often been admitted because of side effects. The contribution of the pharmacist is therefore essential to ensure safe and effective treatment with minimal interactions and side effects. Patients under the care of different specialists required interventions with different level of “significance” because of the complexity of the drug treatment. Pharmacists of higher grades were allocated to more complex wards according to their expertise and were able to provide more complex (or significant) interventions to patients with more complex disease states and regimens. This finding backs up previous studies in which more experienced pharmacists were appropriately allocated to wards considered more drug intensive.19
Pharmacists can contribute effectively to patient care only if they are present and given enough time for their visits. This is demonstrated by the low number of interventions recorded during the visits to the outpatient day units. Patients may receive suboptimal care if not seen by a pharmacist.7–14,18 This has implications for workforce planning, allocation of appropriate levels of staff to appropriate cases and education and training of specialist and higher level practitioners.
The problems requiring interventions highlight areas that need improvement in prescribing at both the CPU and on the wards. At the CPU, pharmacists often dealt with chemotherapy prescriptions with incorrect or missing information. This is of concern as cancer drugs are prepared for individual patients so information should be available to enable individual dosing and constant monitoring of the patient’s health status.18 At the CPU, changes in a patient’s health status commonly resulted in adjustments to therapy. This demonstrates the importance of the clinical knowledge of the pharmacist in production. On the wards, the drug charts often lacked clerical information, such as the allergy status of the patient, and information on the dosage regime of the prescribed drugs. Additionally, drugs prescribed as support care often did not cover the indications, or the treatment was prolonged without obvious reason. There is a role for pharmacists to prescribe (as supplementary and independent prescribers) to ensure appropriate and timely prescribing for these complex patients.
Medical practitioners agreed with most of the proposed interventions, which confirms other research that pharmacy input is needed for high-quality care in cancer.8–14,23 The interventions recorded at the CPU were rated as more significant than those on the wards. Drug-related problems requiring interventions of minor significance may not have occurred at the CPU for several reasons. It may be that the risk management mechanisms in place in the dispensing of chemotherapy doses have minimised the need for “minor intervention”, or such problems may not have reached the CPU because of the collaboration between pharmacists and medical practitioners on the wards. This highlights the advantage of integrated clinical and technical pharmacy services on the cancer wards and at the CPU.
Conclusion
The study evaluated the interventions made by pharmacists in the care of cancer patients. The interventions improved the standard of patient care and prevented events that could have led to patient harm. The study confirms the need for pharmacists at different levels and with different expertise to meet the needs of different patient groups. When planning our workforce, we need to consider the knowledge and skills required for the care of different patient groups and the associated risks of their prescribed regimens. In this way, we can plan services to best meet the needs of patients while utilising the workforce most effectively.
Acknowledgements
Lea Knez received a studentship under the European Leonardo programme. We thank Samo Rozman for his role in developing the data collection form, piloting the data collection and collecting the data. We also thank all the pharmacists who participated in this study, the members of the expert panel and the medical oncologist. Finally, we thank Ales Mrhar for his contribution to the study.
About the authors
Lea Knez, Magister Farmacije, is research pharmacist, academic department of pharmacy, Barts and The London NHS Trust. Raisa Laaksonen, PhD, MRPharmS, is lecturer in pharmacy practice, department of pharmacy and pharmacology, University of Bath. At the time of the study she was research fellow, academic department of pharmacy, Barts and The London NHS Trust. Catherine Duggan, PhD, MRPharmS, is associate director of clinical pharmacy for evaluation and development, East and South East England Specialist Services, and senior clinical lecturer, School of Pharmacy, University of London. At the time of the study she was director, academic department of pharmacy, Barts and The London NHS Trust. Rajinder Nijjar, ClinDipPharm, MRPharmS, is senior directorate pharmacist — cancer services, Barts and The London NHS Trust.
Correspondence to: Raisa Laaksonen, Department of Pharmacy and Pharmacology, University of Bath, Bath BA2 7AY (e-mail r.laaksonen@bath.ac.uk)
References
- Department of Health. The new NHS: modern, dependable. London: HM Stationery Office; 1997.
- Department of Health. A first class service: quality in thenew NHS. London: HM Stationery Office; 1998.
- Department of Health. The NHS plan. London: Stationery Office; 2000.
- Department of Health. Pharmacy in the future —implementing the NHS plan. London: Department of Health;2000.
- Department of Health. A vision for pharmacy in the new NHS. London: Department of Health Publications; 2003.
- Audit Commission. A spoonful of sugar: medicines management in NHS hospitals. London: Audit Commission;2001.
- Department of Health. Pharmacy workforce in the new NHS.London:Department of Health Publications; 2002.
- Burtonwood AM, Hinchliffe AL, Tinkler GG. A prescription for quality: a role for the clinical pharmacist in general practice. Pharmaceutical Journal 1998;261:678–80.
- Eadon H. Assessing the quality of ward pharmacists’ interventions. International Journal of Pharmacy Practice1992;1:145–7.
- Granas AG, Bates I. The effect of pharmaceutical review ofrepeat prescriptions in general practice. International Journal of Pharmacy Practice 1999;7:264–75.
- Krass I, Smith C. Impact of medication regimen reviews performed by community pharmacists for ambulatory patients through liaison with general medical practitioners. International Journal of Pharmacy Practice 2000;8:111–20.
- Price RN, Rogers A. Intervention monitoring on admissions wards. Hospital Pharmacist 2000;7:81–4.
- Serracino-Inglott A, Azzopardi LM, Adami MZ. Pharmacist intervention reduces side effect. ASHP Midyear Clinical Meeting. 2001;36:445E.
- Westerlund T. Drug-related problems. Identification, characteristics and pharmacy interventions [thesis]. Göteborg University: Department of Social Medicine; 2002.
- McLean W, Poston J, Tsao S. Experience with external review panels to validate a large clinical pharmacy intervention study. Canadian Journal of Hospital Pharmacy 1998;51:200–8.
- Department of Health. The NHS improvement plan. Putting people at the heart of public services. London: Stationery Office; 2004.
- Cancer Research UK. Cancer statistics. Available at http://info.cancerresearchuk.org/cancerstats/incidence/?a =5441 (accessed November 2007).
- Department of Health. The NHS cancer plan: a plan for investment, a plan for reform. London: Department of Health; 2000.
- Barber N, Batty R, Ridout DA. Predicting the rate of physician-accepted interventions by hospital pharmacists in the United Kingdom. American Journal of Health-System Pharmacy 1997;54: 397–405.
- Department of Health. An organisation with a memory. London: HM Stationery Office; 2000.
- Royal Pharmaceutical Society of Great Britain. Achieving excellence in pharmacy through clinical governance. London: The Society; 1999.
- Allan EL, Barker KN. Fundamentals of medication error research. American Journal of Hospital Pharmacy 1990;47:555–71.
- Hawkey CJ, Hodgon S, Norman A, Daneshmen TK, Garner ST. Effect of reactive pharmacy intervention on quality of hospital prescribing. BMJ 1990;300:986–90.
- Mackie CA. Randomised controlled trial of medication review [thesis]. Glasgow: University of Stratchlyde; 2002.
- Van Mill JWF, Westerlund LOT, Hersberger KE, Schaefer MA. Drug-related problem classification systems. Annals of Pharmacotherapy 2004;38:859–67.
- Hussain S. An evaluation of dispensing and administration of medicines in secondary care [thesis]. London: School of Pharmacy; 2005.
- Tessier A, Bedouch P, Allenet B, Brudieu B, Calop J, et al. Computerized prescriber order entry associated with pharmacit participation in physician round in a French teaching hospital: assessment of pharmacist’s interventions. ASHP Summer Meeting, 2005;62:P8E.
