Key points
- Early, intensive glycaemic control reduces the onset and progression of vascular disease.
- All newer agents have no increased cardiovascular risk compared with usual care.
- Canagliflozin, empagliflozin, liraglutide and the once-weekly formulation of semaglutide, reduced CV risk compared with usual care.
Introduction
In less than a century, diabetes has moved from being a disease associated with premature death to being a long-term condition. Today around 451 million adults worldwide have diabetes (>90% of whom have type 2 diabetes [T2DM]), with this number projected to increase to around 693 million by 2045[1]
.
It is imperative that the glucose-lowering drugs being developed are safe, effective and do not cause or exacerbate diabetes-associated conditions. Herein, consideration is given to the evolution of treatment in T2DM, and the recent multitude of cardiovascular outcome trials (CVOTs) with glucose-lowering drugs.
Sources and selection criteria
The majority of articles discussed were identified during the authors’ previous database searches, but updated and supplemented using articles from PubMed/MEDLINE and online journals with a focus on CVOTs with glucose-lowering agents. The early trials, as well as the main CVOTs and key publications, are included to support the discussion of controversies and subsequent trial interpretation. Other studies included provide a rationale for the current ongoing CVOTs.
The evolution of diabetes therapeutics
Diabetes has been recognised for millennia; however, prior to the 20th century, treatment options were limited to herbal remedies and/or lifestyle approaches, including diets that essentially restricted carbohydrate consumption and were generally unsustainable[2]
. The advent of insulin therapy in the 1920s extended for the lives of people with type 1 diabetes (T1DM), and helped to further differentiate between T1DM and T2DM. The mid-20th century saw the arrival of oral glucose-lowering agents to treat T2DM: the sulphonylurea tolbutamide in 1956, and the biguanide metformin in 1957. The sulphonylurea class became the cornerstone of oral glucose-lowering therapy for around 30 years (see Figure 1). Although the life expectancy of patients with diabetes improved, this came at a cost of increased morbidity — notably consequent to microvascular and macrovascular disease[3]
.
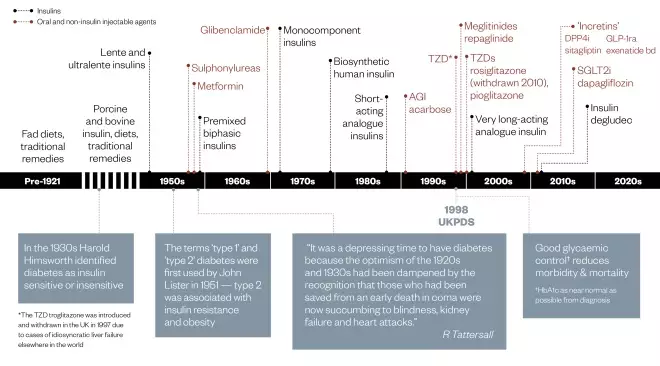
Figure 1: Diabetes treatment timeline
AGI = alpha-glucosidase inhibitor; TZD = thiazolidinedione; SGLT2i = sodium-glucose co-transporter-2 inhibitor; UKPDS = United Kingdom Prospective Diabetes Study; DPP4i = dipeptidyl peptidase 4 inhibitor; GLP-1ra = glucagon-like peptide 1 receptor agonist
Early trials
The University Group Diabetes Program (UGDP), the first large, randomised, double-blind, placebo-controlled trial to investigate if glucose-lowering therapy could prevent or delay the progression of cardiovascular (CV) disease in people with T2DM commenced in 1961. Around 200 patients were included in each study arms of: insulin (variable dose), insulin (fixed dose), tolbutamide or placebo; and a phenformin arm was added later. The tolbutamide arm of the UGDP showed an excess of CV deaths — notably among those individuals with the poorest glycaemic control. However, it should be remembered that patients were treated to relieve symptoms (ideally eliminating glycosuria), since glycaemic targets had yet to arrive. The biguanide phenformin was introduced in the United States during the UGDP study, but withdrawn in 1978 owing to an unacceptable risk of fatal lactic acidosis[4]
.
In the 1960s, phenformin was the biguanide of choice, so use of metformin was not considered in the United States[5]
; metformin did not receive marketing authorisation in the country until December 1994[6]
.
The UK Prospective Diabetes Study (UKPDS) aimed to obtain the best treatment strategy for T2DM and, unlike the UGDP and subsequent CVOTs, the UKPDS recruited patients with newly diagnosed T2DM and followed them for up to 20 years. In addition to usual care (diet), patients were randomised to treatment with insulin or sulphonylureas (chlorpropamide or glibenclamide) or metformin (in overweight patients)[7]
. Treatment was increased as needed to achieve or maintain a lower glycaemic target (HbA1c≤7% [54mmol/mol]) in the intensively treated group, compared with usual care (HbA1c≤7.9% [63mmol/mol]). Measurement of glycation of the N-terminal valine of the B-chain of adult haemoglobin — namely HbA1c— became clinically available in the late 1970s and subsequently referencing standards were aligned with those used in the Diabetes Control and Complications Trial (DCCT). Following an interim of dual unit reporting between 2009–2011, the UK now only reports in the International Federation of Clinical Chemistry and Laboratory Medicine’s preferred referencing of mmol/mol. Nevertheless, ongoing and newly initiated international trials report in DCCT-aligned percentages[8]
.
The UKPDs was a turning point. It showed that intensive glycaemic control (maintaining glycaemia as near normal as possible) from diagnosis of T2DM reduced premature death and vascular morbidity, and these benefits were evident even if control deteriorated in subsequent years — the so-called ‘legacy effect’[9],[10]
. It is noteworthy that the CV benefits of good initial glycaemic control may not become evident for many years. The UKPDS demonstrated the benefits of good blood pressure control, confirmed the progressive degeneration of glycaemic control in T2DM and provided a rationale for glycaemic targets[7],[10],[11]
.
When the Steno-2 study was designed in 1990, the UKPDS was ongoing and the evidence base for the treatment of diabetes was virtually non-existent. The association of components of the metabolic syndrome (the clustering of CV disease risk factors), the underlying pathophysiology of which is aetiologically underpinned by insulin resistance, had introduced polypharmacy to the T2DM treatment regimen; however, the effect of intensive multifactorial risk factor management was unknown[12]
. The introduction of statins, angiotensin-converting-enzyme inhibitors and angiotensin-receptor blockers in the 1980s, and use of aspirin for primary prevention of myocardial infarction (MI), offered greater opportunity for intensive risk factor management. When the results of Steno-2 were reported in 2003, it showed that intensive multiple risk factor management for 7.8 years reduced the relative risk of CV disease and development or progression of microvascular disease by more than 50%[12]
. The HbA1c target (<6.5%, 48mmol/mol) was the most difficult to achieve, being attained by only 15% of patients, whereas >40% achieved their systolic blood pressure target (<130mmHg) and >70% achieved their cholesterol target (<4.5mmol/l)[12]
.
Macrovascular disease continues to be a major cause of morbidity and mortality[13],[14]
. In the 1980s, the life expectancy of middle-aged people with T2DM was reduced by 5–10 years, with the majority of deaths caused by CV disease: death from heart disease and stroke was 2–4 times higher than in the non-diabetic patient population[13]
. Today, even with effective management of multiple CV risk factors, death occurs on average six years earlier than in a person without diabetes, and diabetes doubles the risk of morbidity and death from vascular diseases[14]
.
Next-generation trials
In the UKPDS, intensive versus usual glycaemic control (6–20 years for a median of 10 years) reduced MI by 16%, but this was of borderline significance (P =0.052), and prompted the inception of several large prospective trials to investigate the effect of intensive glycaemic control on major adverse cardiovascular events (MACE)[7]
. Unlike UKPDS, the next generation trials (Action to Control Cardiovascular Risk in Diabetes [ACCORD], Action in Diabetes and Vascular disease [ADVANCE] and Veterans Administration Diabetes Trial [VADT]), operated on the premise that it is never too late to introduce intensive glycaemic control[15],[16],[17]
. Intensification, therefore, occurred in older patients with longstanding, often poorly controlled diabetes, many of whom had already experienced a CV event. Each of these studies showed that improving glycaemic control reduced the onset and delayed the progression of microvascular disease, but the effect on macrovascular disease was not as clear-cut[15],[16],[17]
. In ACCORD there was a decrease in the number of non-fatal MIs, but an increase in CV mortality leading to early termination of the study[15]
. However, these deaths mainly occurred in patients with a history of poorer glycaemic control who had failed to respond to therapy intensification[15]
. Interestingly, VADT also showed more MACE in patients with a history of poor glycaemic control, while affirming the benefits of achieving good glycaemic control as early as possible after diagnosis[17]
. These studies re-ignited the HbA1c ‘how low to go’ debate, especially in older patients at high CV risk, and heralded a return to the pre-protocol era of individualisation.
Reassuringly, a meta-analysis of ACCORD, ADVANCE, VADT and UKPDS published in 2009 (using five-year data to be consistent with the shorter duration of the other studies) demonstrated the benefit of intensive glycaemic control in all studies, including a decrease in MI (15%) and all CV events (9%)[18]
.
Increasing cardiovascular risk
The UKPDS esÂtablished that good glycaemic control cuts complications; but, there have long been suggestions that glucose-lowering agents may have additional actions that alter CV risk[4],[19]
. Sulphonylureas have been subject to debate since the UGDP, but subsequent trials have not confirmed detrimental effects of sulphonylurea therapy on CV outcomes when these agents are used appropriately (i.e. hypoglycaemia or excess weight gain are avoided). The recent Thiazolidinediones or Sulphonylureas and Cardiovascular Accidents Intervention Trial (TOSCA-IT; NCT00700856), which was terminated early following a futility analysis, showed no difference in CV outcomes when comparing treatment with pioglitazone (n=1,535) or a sulphonylurea (n=1,493; gliclazide 50%, glimepiride 48%, glibenclamide 2%) as an add-on to metformin for a median of 4.8 years. In addition, the rates of heart failure, bladder cancer and fractures — issues of concern with pioglitazone therapy — did not differ between the treatment groups; weight gain was similar (around 2kg), but more patients had hypoglycaemia in the sulphonylurea (34%) than pioglitazone (10%) treatment group (P <0.0001). Fewer patients receiving pioglitazone required insulin rescue therapy during the study (11% versus 16% P <0.0001), suggesting that greater durability of glycaemic control can be achieved with pioglitazone as add-on to metformin[20]
.
Metformin may have beneficial effects beyond lowering glucose — indeed the metformin summary of product characteristics (SPC) notes a reduction in morbidity and mortality, including a significant reduction in the absolute risk of MI, in T2DM. Pre-clinical and clinical studies have also shown positive influences on a range of CV risk factors[21]
.
The Reducing With Metformin Vascular Adverse Lesions in Type 1 Diabetes (REMOVAL) study (NCT01483560), aimed to test whether three years’ treatment with metformin added to titrated insulin therapy reduces atherosclerosis. The study was undertaken in adults (mean age 55.5 years; body mass index 28.5; HbA1c 8.05%) with T1DM for a mean duration of 33.8 years who had ≥3 CV risk factors (73% were on antihypertensive medication, 82% were taking statins and around 12% had a documented CV disease). The addition of metformin for three years reduced the progression of atherosclerosis, with the rates of change of mean and maximal carotid intima-media thickness around half of those of patients on placebo. Metformin reduced weight by about 1.2kg (P <0.001), insulin dose by around two units/day and low-density lipoprotein by 5mg/dl;0.13mmol/l. HbA1c was lowered at three months (P <0.0001), but significance was lost thereafter and there was no increase in hypoglycaemia. Unexpectedly, there was a small improvement in estimated glomerular filtration rate (eGFR) during the first three months of treatment, and significance was retained at three years regardless of eGFR methodology: modification of diet in renal disease (P <0.0001), Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)creatinine (P =0.0002) and CKD-EPIcystatin C (P =0.011)[22],[23]
.
Thiazolidinediones are known to cause fluid retention and are, therefore, contraindicated in heart failure in Europe (New York Heart Association [NYHA] functional classification stages I–IV), but only for NYHA stages III and IV in the United States. However, in the European phase III study Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive), pioglitazone significantly (P =0.02) decreased the composite secondary end-point of MACE (all-cause mortality, non-fatal MI and non-fatal stroke) in older T2DM patients at high CV risk. A recent meta-analysis of nine trials supports the view that pioglitazone reduces the risk of MACE in T2DM[24]
. In 2007, a meta-analysis of 42 studies was published that drew media attention; the pros and cons of this publication, which showed that rosiglitazone increased the risk of MI (P =0.03) and hinted that it might increase CV-related death (P =0.06), are elucidated in an accompanying editorial[25]
. At this time, the large multinational phase III trial, Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD), investigating the impact of rosiglitazone on CV outcomes was ongoing. Despite the media and regulatory furore, this trial was allowed to continue. An interim analysis was undertaken that did not support the findings of the meta-analysis[26]
; nevertheless, in 2010 rosiglitazone was withdrawn in Europe and use was restricted in the United States under a risk evaluation and mitigation strategy (REMS) programme with a requirement for independent re-adjudication of the final RECORD data. The REMS was also put in place by the United States Food and Drug Administration (FDA), the governmental department responsible for evaluating the safety and efficacy of medicines in the United States. Under the REMS programme, patients could continue on rosiglitazone, but it could only be initiated in patients in extenuating circumstances. Physicians had to be trained and registered as rosiglitazone prescribers and pharmacies had to be specially registered to dispense the drug.
On completion, RECORD had confirmed that rosiglitazone does not increase the risk of overall CV morbidity or mortality, but the data were inconclusive regarding any effect of the drug on MI[27]
. In November 2013, following another independent re-adjudication and regulatory interrogation of RECORD — including a multiplicity of additional statistical analyses — the FDA concurred that rosiglitazone did not increase CV risk and lifted some of the REMS restrictions[28]
. Since May 2014, all retail pharmacies in the United States have been able to dispense rosiglitazone-containing drugs and in December 2017 the FDA eliminated the REMS for medicines containing rosiglitazone[29]
.
Cardiovascular confidence
The series of reported incidents related to rosiglitazone prompted an FDA announcement in December 2008 that a meta-analysis of CV events during phase II and phase III studies must be included in all new drug application submissions for glucose-lowering therapies (see Figure 2)[30]
. Large phase IV trials have almost become culturally mandatory, with no company wanting to risk disadvantaging their drug with the absence of a post-marketing CV trial, despite having met FDA criteria. Indeed, several more recent phase III studies have been designed to specifically address CV risk, with many destined to continue into the post-marketing phase. It should be remembered that these CVOTs are safety studies and it is a bonus when they provide outcome data on other issues of interest, such as risk of neoplasms, pancreatitis and hypoglycaemia; bone health; weight change; neurological and renal function; durability of glycaemic control; other metabolic indices; adherence and, to add an experimental twist, post hoc analysis of ‘unknown unknowns’ that may be exposed by the trial. However, conducting a CVOT is very costly and may divert resources from further mechanistic investigation of the agent, as well as compromising investment in research into novel agents in early development.
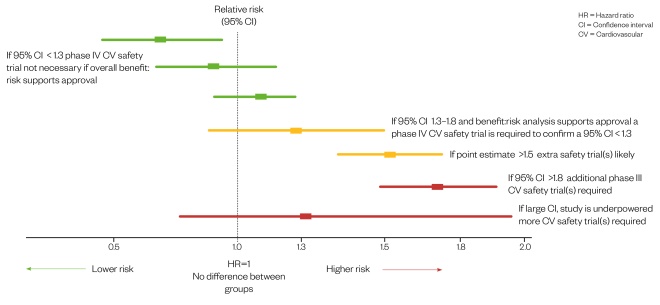
Figure 2: Meta-analysis of phase II/III studies for potential cardiovascular risk[30]
Source: United States Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for industry diabetes mellitus — evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes.
Are all CVOTs equal?
CVOTs aim to demonstrate that the addition of the novel agent is as safe as usual care (i.e. non-inferior with regard to CV outcomes [at least three-point MACE: a composite of CV death, non-fatal MI or non-fatal stroke] and, perchance, offer additional benefit [superiority]). Trials have aimed for glycaemic equipoise to ensure that outcomes cannot be attributed to the glucose-lowering efficacy of the novel drug. So does this approach offer opportunities for direct comparisons between trials?
In the current era of primary multiple risk factor management, CV event rates are very low in patients with T2DM who lack CV disease. CVOTs are mainly event-driven, and the inclusion of subjects with coexisting CV disease or proteinuria increases event rates about 4–16-fold[31]
.Thus including subjects at higher CV risk permits trials to be conducted with fewer subjects and over a shorter duration; but shorter trials have worse CV outcomes than longer-term studies — with short-term outcomes often being consequent to pre-trial treatment, rather than the agent under investigation. The baseline characteristics of patients and main CV outcomes in the CVOTs which have reported by (September 2017) are summarised in Table 1.
Table 1: Summary of patient characteristics and main cardiovascular outcomes
[32],[33],[34],[35],[36],[37],[38],[39],[40],[41]
The Freedom CVOT (ITCA 650) was completed in March 2016, after 1.2 years’ follow-up, but at time of writing its results have not been published in the medico-scientific literature. The study involved a novel implantable osmotic pump device (Medici Drug Delivery SystemTM; Intarcia Therapeutics, Inc. [Boston, MA]) for continuous, consistent, subcutaneous release of exenatide for at least six months. As Intarcia submitted a new drug application in November 2016 (accepted by FDA in February 2017); therefore, it is safe to assume that ITCA 650’s four phase III studies must have met their primary and secondary end-points, which include non-inferiority for three-point MACE and hospitalisation for angina[42]
. Several CVOTs are ongoing and their main features are listed in Table 2.
The CVOTs have varying proportions of subjects at differing extents of higher CV risk, different estimations of event rates and trial duration, a variety of primary and secondary end-points, a diversity of statistical and hierarchical analyses, and inclusion of a placebo and/or active comparator (in addition to the multifarious approaches to usual care) which make direct comparisons between trials difficult. This is often exacerbated by results being presented in formats that seem designed to obfuscate comparison.
| Trial [www.clinicaltrials.gov ID] | Dates | Follow-up (years approximate) | Number of patients | Cardiovascular type | Primary end-point | |
|---|---|---|---|---|---|---|
CKD: chronic kidney disease; CV: cardiovascular; CVD: coronary, cerebro and peripheral vascular disease; DPP4i: dipeptidylpeptidase 4 inhibitor; GLP -1 ra: glucagon -like peptide-1 receptor agonist; HHF: hospitalisation for heart failure; 3pt MACE: Major adverse cardiac events (composite of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke); SGLT2i: sodium glucose co-transporter 2 inhibitor; UACR: urine albumin-to-creatinine ratio. | ||||||
| DPP4i | CAROLINA Linagliptin vs glimepiride [NCT01243424] | 2010–2019 | 8 | 6,072 | ≥40 years + CVD or end-organ damage, or ≥70 years, or ≥2 CV risk factors | 3pt MACE |
| CARMELINA Linagliptin [NCT01897532] | 2013–2017 | 4.5 | Approximately 7,003 | ≥18 years + micro-/macro-albuminuria + CVD, and/or CKD (pre-defined UACR) | 3pt MACE | |
| GLP -1 ra | REWIND Dulaglutide [NCT01394952] | 2011–2018 | 6.5 | 9,622 | ≥50 years + CVD, or ≥55 years and + subclinical vascular disease, or ≥60 years + ≥2 CV risk factors | 3pt MACE |
| HARMONY Albiglutide [NCT02465515] | 2015–2018 | 4 | 9,400 | ≥40 years + CVD | 3pt MACE | |
| PIONEER 6 Semaglutide (oral) [NCT02692716] | 2017–2018 | 1.5 | 3,176 | ≥50 years + CVD, or ≥60 years and ≥1 CV risk factor | 3pt MACE | |
| SGLT2i | DECLARE Dapagliflozin [NCT01730534] | 2013–2019 | 6 | 17,276 | ≥40 years + high CV risk | 3pt MACE — Co-primary: CV death or HHF |
| VERTIS CV Ertugliflozin [NCT01986881] | 2013–2019 | 6 | 8,000 | ≥18 years + CVD | 3pt MACE | |
Outcomes of CVOTs
All CVOTs completed at time of writing have achieved their primary end-point of non-inferiority with regard to CV risk. However, some trials (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results [LEADER], Trial to Evaluate Cardiovascular and other long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes [SUSTAIN 6], Canagliflozin Cardiovascular Assessment Study [CANVAS] and Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients [EMPA-REG]) have also shown CV superiority compared with usual care[37],[38],[39],[40]
.
The CVOTs are safety trials, and it is reassurig that outcomes to date support CV safety — especially in T2DM patients at high CV risk. However, the proportion of patients at such high CV risk is much lower in the general diabetes population; thus trials that include lower proportions of patients at high CV risk may be considered more representative of the outcomes to be expected in ‘real world’ practice. The CV results of CVOTs (to September 2017) are summarised in Table 2.
The CVOTs have produced additional data of interest, for example, the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) trial raised an index of suspicion with a small but significant increase in hospitalisation for heart failure in patients taking saxagliptin (hazard ratio [HR]: 1.27), which appeared to be corroborated by a non-significant increase (HR: 1.07) in the Examination of Cardiovascular Outcomes: Alogliptin versus Standard of Care (EXAMINE) trial[33],[34]
; however, the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) delivered a HR of 1.00[32]
. The CVOT for omarigliptin [NCT01703208] — a once-weekly dipeptidyl peptidase-4 (DPP4) inhibitor, used to treat T2DM in Japan — was terminated early when it was decided not to submit for a marketing application in the United States. The results of this study, however, had already shown that treatment with omarigliptin for a median 1.8 years did not increase the risk of MACE or hospitalisation for heart failure[43]
.
These trial results raise questions about CVOT design: the subjectivity of clinical decision making for determination of hospitalisation; the geographical and logistical practicalities of accessing hospital treatment (e.g. hospitalisation) is less likely in patients in rural areas. Different studies do not lay the same emphasis on a particular outcome — in CANVAS, for example, amputations were a pre-specified adverse event[44]
. The unexpected increase in amputations in canagliflozin-treated patients (P <0.001) prompted a re-examination of amputations in EMPA-REG. Interestingly, there were 6.3 amputations/1,000 patient years in both the canagliflozin- and empagliflozin-treated groups and in the EMPA-REG placebo group, but only 3.4 amputations/1,000 patient years in the CANVAS placebo group[45]
. A database analysis of more than 118,000 new users of sodium-glucose co-transporter-2 inhibitors (SGLT2i) and 226,623 patients on non-SGLT2i glucose-lowering drugs showed an amputation incidence of 1.22 and 1.87 events/1,000 patient years, respectively. Among patients taking an SGLT2i, the incidence of amputation with canagliflozin, dapagliflozin and empagliflozin was 1.26, 0.96 and 1.39 events/1,000 patient years, respectively. Thus, this real-world study in a broad population of T2DM patients does not suggest that SGLT2i therapy increases amputation risk[46]
.
While there is suspicion that negative outcomes from one trial may be applied to a class, there is hope that positive results may herald a class effect. To date, the SGLT2i CVOTs EMPA-REG (empagliflozin) and CANVAS (canagliflozin) have shown a reduction in hospitalisation for heart failure (HR: 0.65 and 0.67, respectively)[39],[40]
. The Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL) study database has shown that treatment with an SGLT2i versus usual oral glucose-lowering drug therapy significantly (P <0.001) reduced heart failure and death in patients with and without established CV disease[47]
.
Fears of a potential association between use of incretin-based therapies and increased pancreatic issues have been allayed by the CVOTs, while supporting the caution of avoiding DPP4 inhibitor and GLP1ra therapy in patients with a history of alcohol abuse, pancreatitis or pancreatic cancer[32],[33],[34],[35],[36],[37],[38]
. The significant increase (HR: 1.72) in retinopathy complications observed in SUSTAIN 6 (semaglutide, injection once weekly) has subsequently been shown as consequent to a very rapid improvement in glycaemic control in affected patients — 83% of whom had retinopathy at baseline[48]
.
The Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events (DEVOTE) is the first double-blind CVOT with a head-to-head of two basal insulins (insulin degludec versus insulin glargine U100) added to usual care of T2DM patients at high CV risk. In addition to degludec demonstrating CV non-inferiority, 27% fewer patients experienced severe hypoglycaemia and there was a 53% lower rate of nocturnal severe hypoglycaemia (P <0.001 versus glargine)41. Interestingly, in the earlier Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial, addition of the basal insulin glargine to usual care for a median of 6.2 years (to achieve normal fasting plasma glucose levels) did not alter CV risk compared with target-driven usual care in people with T2DM (HR: 1.02 for three-point MACE)[49]
.
Currently, the Cardiovascular Outcome Study of Linagliptin versus Glimepiride in Patients With Type 2 Diabetes (CAROLINA; NCT01243424) is the only other head-to-head CVOT; results are expected in early 2019.
Conclusion
This review illustrates how the history of non-insulin glucose-lowering agents (both oral and injectable) has been plagued by concerns of CV safety. Trials specifically designed to assess CV safety have been conducted (or are ongoing) for the newer glucose-lowering therapies, and have reassuringly shown no deleterious effects on a selection of CV functions (with the exception of hospitalisation for heart failure in SAVOR). These data have been used to inform guidance (such as the National Institute for Health and Care Excellence’s ‘Type 2 diabetes in adults: management NICE guideline [NG28]’)[50]
. Several of the trials (REMOVAL, LEADER, SUSTAIN 6, EMPA-REG and CANVAS) have revealed significant benefits of these agents in reducing MACE and additionally providing some protection against deteriorating renal function in T2DM — an aspect requiring further investigation.
As the T2DM epidemic continues to gather pace, these new CVOTs provide confidence that early and effective pharmacological intervention to control blood glucose can safely alleviate acute symptoms, defer and reduce microvascular complications, and additionally provide benefits against the CV diseases that so often signal premature mortality in people with T2DM.
Author disclosures and conflicts of interest:
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. Caroline Day has previously undertaken educational programmes and ad hoc advisory activities sponsored by pharmaceutical companies, but these do not compromise the submitted work. No writing assistance was used in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click: “Planned Learning”
If your learning was spontaneous, please click: “Unplanned Learning”
References
[1] International Diabetes Federation. IDF Diabetes Atlas 8th Edn 2017. Available at: www.diabetesatlas.org/ (accessed April 2018)
[2] Day C & Bailey CJ. The hypocaloric diet in type 2 diabetes — déjà vu. Br J Diabetes Vasc Dis 2012;12:48–51. doi: 10.1177/1474651412437503
[3] Tattersall R. The dark ages of diabetes. Br J Diabetes Vasc Dis 2002;2:423–426. doi: 10.1177/14746514020020060201
[4] Kilo C, Miller J Ph & Williamson JR. The crux of the UGDP. Diabetologia 1980;18:179–185. doi: 10.1007/BF00251913
[5] Howlett HCS & Bailey CJ. Introduction of metformin into clinical practice: the French innovation. In Baily CJ, Campbell IW, Chan JCN et al. (Eds). Metformin: The Gold Standard. 2007;17–22. John Wiley & Sons Ltd, Chichester, UK.
[6] Bailey CJ. Metformin: historical overview. Diabetologia 2017;60(9):1566–1576. doi: 10.1007/s00125 -017-4318-z
[7] UK Prospective Diabetes Study (UKPDS) group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853. doi: 10.1016/S0140 -6736(98)07019-6
[8] Day C & Bailey CJ. HbA1c— changing units. Br J Diabetes Vasc Dis 2009;9(3):134–136. doi: 10.1177/1474651409106110
[9] Stratton IR, Adler AI, Neil HAW et al. Association of glycaemia with macrovascular and microvascular complications of Type 2 diabetes: prospective observational study. BMJ 2000;321:405–412. doi: 10.1136/bmj.321.7258.405
[10] Holman RR, Sanjoy KP, Bethel MA et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470
[11] UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713. doi: 10.1136/bmj.317.7160.703
[12] Gaede P, Lund-Andersen H, Parving H-H & Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591. doi: 10.1056/NEJMoa0706245
[13] Geiss LS, Herman WH & Smith PJ. Mortality in non-insulin dependent diabetes. In Diabetes in America 2nd Edn 1995;233–257. National Institutes of Health, NIDDK, NIH Publications No 95-1468, Washington, United States.
[14] Seshasai SRK, Keptoge S, Thompson A et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841. doi: 10.1056/NEJMoa1008862
[15] The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743
[16] The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:560–572. doi: 10.1056/NEJMoa0802987
[17] Duckworth W, Abraira C, Moritz T et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139. doi: 10.1056/NEJMoa0808431
[18] Turnbull F, Abraira C, Anderson RJ et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–2298. doi: 10.1007/s00125 -009-1470-0
[19] UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352;854–865. doi: 10.1016/S0140 -6736(98)07037-8
[20] Vaccaro O, Masulli M, Nicolucci A et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. 2017;5(11):887–897. doi: 10.1016/S2213 -8587(17)30317-0
[21] Campbell IW & Howlett HCS. Metformin and the heart. In Campbell IW et al. Eds. Metformin: 60 years of clinical experience. 2017;45–57. WILEY-VCH Weinheim, Germany.
[22] Petrie J R, Chaturvedi N, Ford I et al ., for the REMOVAL Study Group. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial.Lancet Diabetes Endocrinol 2017;5(8):597–609. doi: 10.1016/S2213 -8587(17)30194-8
[23] Rossing P. Metformin and the kidney in type 1 diabetes. In REMOVAL: a randomized controlled trial of metformin in adults with type 1 diabetes. EASD, 10 September 2017. Available at: https://www.easd.org/removal.html (accessed April 2018)
[24] Liao H-W, Saver JL, Wu Y-L et al. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open 2017;7(1):e013927. doi: 10.1136/bmjopen-2016-013927
[25] Psaty BM & Furberg CD. Rosiglitazone and cardiovascular risk. N Engl J Med 2007;356:2522–2524. doi: 10.1056/NEJMe078099
[26] Home PD, Pocock SJ, Beck-Nielsen H et al., for the RECORD Study Group. Rosiglitazone evaluated for cardiovascular outcomes — an interim analysis. N Engl J Med 2007;357:28–38. doi: 10.1056/NEJMoa073394
[27] Home PD, Pocock SJ, Beck-Nielsen H et al ., for the RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373(9681):2125–2135. doi: 10.1016/S0140 -6736(09)60953-3
[28] FDA Drug Safety Communication. FDA requires removal of some prescribing and dispensing restrictions for rosiglitazone-containing diabetes medicines. 2013. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm376389.htm (accessed April 2018)
[29] FDA Drug Safety Communication. Rosiglitazone-containing Diabetes Medicines. Drug Safety Communication - FDA Eliminates the Risk Evaluation and Mitigation Strategy (REMS). Available at: https://www.fda.gov/downloads/drugs/drugsafety/ucm477575.pdf (accessed April 2018)
[30] United States Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry diabetes mellitus — evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008. Available at: https://www.fda.gov/downloads/Drugs/Guidance%20ComplianceRegulatoryInformation/Guidances/ucm071627.pdf (accessed April 2018)
[31] Preiss D, Sattar N & McMurray JJ. A systematic review of event rates in clinical trials in diabetes mellitus: the importance of quantifying baseline cardiovascular disease history and proteinuria and implications for clinical trial design. Am Heart J 2011;161:210–219.e1. doi: 10.1016/j.ahj.2010.10.019
[32] Green JB, Bethel MA, Armstrong PW et al., for the TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352
[33] White WB, Cannon CP, Heller SR et al, for the EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889
[34] Scirica BM, Bhatt DL, Braunwald E et al ., for the SAVOR -TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684
[35] Holman RR, Bethel MA, Mentz RJ et al ., for the EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917
[36] Pfeffer MA, Claggett B, Diaz R et al ., for the ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225
[37] Marso SP, Daniels GH, Brown-Fransden K et al., for the LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322. doi: 10.1056/NEJMoa1603827
[38] Marso SP, Bain SC, Consoli A et al., for the SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141
[39] Neal B, Perkovic V, Mahaffey KW et al., for the CANVAS Progam Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. doi: 10.1056/NEJMoa1611925
[40] Zinman B, Wanner C, Lachin JM et al. for the EMPA -REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720
[41] Marso SP, McGuire DK, Zinman B et al ., for the DEVOTE Study Group. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med 2017;377:723–732. doi: 10.1056/NEJMoa1615692
[42] Intarcia Therapeutics Inc. Investors and Media. Available at: https://www.intarcia.com/media/ (accessed April 2018)
[43] Gantz I, Chen M, Suryawanshi S et al . A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP -4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2017;16:112. doi: 10.1186/s12933 -017-0593-8
[44] Neal B, Perkovic V, Mahaffey KW et al., on behalf of the CANVAS Program collaborative group. Optimizing the analysis strategy for the CANVAS program: a pre-specified plan for the integrated analyses of the CANVAS and CANVAS-R trials. Diabet Obes Metab 2017;19:926–935. doi: 10.1111/dom.12924
[45] Radhom K et al. eubmitted; reported by Fulcher G, Matthews D, de Zeeuw Wet al. Canagliflozin cardiovascular assessment study CANVAS. Reported at EASD 2017, Lisbon, Portugal. Available at: https://www.easd.org/virtualmeeting/home.html#!resources/canagliflozin -cardiovascular-assessment-study-canvas (accessed April 2018)
[46] Yuan Z, DeFalco FJ, Ryan PB et al . Risk of lower extremity amputations in patients with type 2 diabetes mellitus treated with SGLT2 inhibitors in the United States: a retrospective cohort study. Diabetes Obes Metab 2017;1–8. doi: 10.1111/dom.13115
[47] Kosiborod M, Cavender MA, Fu AZ et al., on behalf of the CVD -REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on SGLT -2 inhibitors versus other glucose-lowering drugs: the CVD -REAL study. Circulation 2017;136:249–259. doi: 10.1161/CIRCULATIONAHA.117.029190
[48] Visboll T. Cardiovascular outcomes with semaglutide in subjects with type 2 diabetes mellitus (SUSTAIN 6). Session 1-AC-SY09 — Update on Cardiovascular Outcomes Trials (CVOTs). Presented at 77th American Diabetes Association Scientific Sessions 2017, California, United States. Available at: http://www.abstractsonline.com/pp8/#!/4297/presentation/7308 (accessed April 2018)
[49] The ORIGIN trial investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. New Engl J Med 2012;367:319–328. doi: 10.1056/NEJMoa1203858
[50] National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline [NG28]. Available at: https://www.nice.org.uk/guidance/ng28 (accessed April 2018)


