It is widely accepted that high-quality health care results from high-quality systems and co-ordinated processes and that when poor-quality care occurs, solutions may usually be found at a systems rather than an individual level.[1]
In assessing quality of care intensive care units (ICUs) have a number of established system-wide measures of quality, including standardised mortality rates adjusted for case mix and adherence to national or local guidelines.[2]
One potential quality measure that has not been previously focused upon within ICU is the timeliness and accuracy of medication allergies documentation. Allergies to medicines are common and range in severity from minor rashes to life-threatening anaphylaxis.[3]
Penicillin allergy alone is reported by 10 per cent of hospital inpatients and although the incidence of true allergy is thought to be lower, at 1 to 2 per cent of the hospital population,[4]
it is a significant problem that can cause death if inadvertent administration occurs.[5],[6]
Interestingly, the National Patient Safety Agency/National Institute of Clinical Excellence guidance alert on medicines reconciliation for adult patients admitted to hospital does not mention allergy status in the list of requirements for confirmation.[7]
There is evidence that the early documentation of allergy status is important for safety in hospitals.[8]
Although it is our hospital’s policy that medicines must not be prescribed, administered or dispensed unless the allergy status is completed and the nature of the allergy recorded,[9]
there are significant potential barriers to this in the ICU, including the patient’s physiological instability, incomplete patient information and the heterogeneity of medical records within our institution (part electronic, part written).[9]
All medical notes and drug prescriptions during a patient’s stay on the ICU within Guy’s and St Thomas’ NHS Foundation Trust (GSTT) are documented on the ICU electronic medical record — Intellivue Clinical Information Portfolio (ICIP) (Phillips). This system is only in use within peri-operative and critical care areas in the trust. Therefore allergy status and drug prescriptions must be transcribed from the paper drug chart used elsewhere in GSTT when a patient is admitted to the ICU. Allergy for all patients may also be recorded in the hospital electronic patient record (EPR) although this is not compulsory on admission.
The initial objectives of this work were to ascertain the adherence to allergy policy within the ICU and to put in place robust processes to improve the timeliness and accuracy of allergy documentation. The second phase was to assess the impact of the interventions.
Three audit standards were devised:
- All patients have their allergy status identical in trust electronic (ICIP and EPR) and paper systems
- All patients have allergy status completed on the ICIP before any drugs (excluding drugs considered essential for emergency administration) are prescribed
- No patients are administered a medicine in the face of a known allergy
Methods
This clinical audit was undertaken in a 30-bedded tertiary referral adult ICU. It was approved by GSTT clinical audit group (registration number 1270). Consent was waived and the audit was unfunded. In the preliminary audit all patients who completed an episode of ICU (admission and discharge) in October 2008 were included to assess the complete patient pathway. The repeat-audit was undertaken in September 2009 after implementation of all recommendations which resulted from the findings of the preliminary audit.
Once patient records were identified, the ICIP record was reviewed. The nature of the allergy, the time of its documentation on the ICIP, its relation to timing of the prescribing and administration of medicines, and its agreement with other sources (EPR and paper medical records) were all recorded. The ICIP was reviewed for prescription and administration of known drug allergens. When potential allergens were given, the ICIP was reviewed for documentation of a risk-benefit decision-making process. Medicines considered essential for emergency administration, including infusions of inotropes and resuscitation drugs, were excluded.
Following the preliminary audit, results were presented to the multidisciplinary teams on the ICU and other wards and at the hospital-wide patient safety forum. A number of recommendations were implemented:
- The ICIP was reconfigured to make the documentation of allergy mandatory within the admission clerking
- The medicines reconciliation process was prioritised, with critical care pharmacists confirming allergies with patient’s notes or family doctor within 24 hours of admission
- The documentation of allergy was highlighted as part of the medical trainee induction process into ICU
Statistical analysis of the effect of recommendations was undertaken using descriptive statistics and a Fisher’s exact test (Microsoft Excel, Microsoft Corp, 2007).
Results
In October 2008, 58 patients completed an episode of critical care, with 13 ICU deaths. In September 2009, 79 patients completed an episode of critical care, with 16 ICU deaths. None of the patients in either data collection period died from anaphylaxis or other allergy to a medicine.
October 2008 (preliminary audit)
Allergy status was completed within 24 hours of ICU admission in 56 of 58 patients (97 per cent). Seventeen patients were documented to have a known drug allergy, of which seven had the nature of the reaction recorded (Table 1).
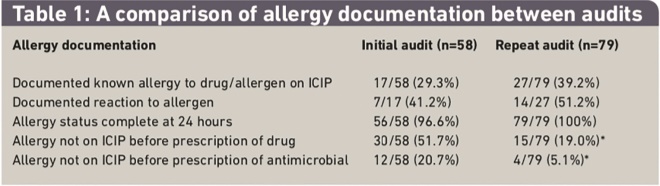
Table 1: A comparison of allergy documentation between audits
Reactions included anaphylaxis (one patient), rash (four patients), neurological impairment due to myasthenia gravis (one patient) and heparin-induced thrombocytopenia (one patient). Four patients had the allergy status documented on admission as “unable to determine”, which was updated to “no known drug allergies” during admission. Drugs were prescribed for 30 patients (52 per cent) before the documentation of allergy, with antimicrobials being prescribed for 12 patients (21 per cent). One patient was administered a potential allergen. No risk-benefit discussion was documented. We were able to obtain written records documenting allergy status for 49 patients before ICU admission and for 37 patients following ICU admission. The allergies documented in the ICIP were in agreement with pre-ICU documentation in 38 of 49 patients’ records (78 per cent) and in agreement with post-ICU documentation in 30 of 37 patients’ records (81 per cent).
The ICIP was in agreement with the EPR for 22 of 32 patients (69 per cent) (Table 2) and the written medical record was in agreement with the EPR for 25 of 32 patients (75 per cent).

Table 2: Agreement between different allergy documentation
September 2009 (reaudit)
All patients had their allergy status recorded within the first 24 hours of ICU admission. Twenty-seven patients were documented to have a known drug allergy, of which 14 had the nature of the reaction recorded (Table 1). Reactions included anaphylaxis or angioedema (four patients) and rash (five patients). Two patients had an allergy status listed as “unable to determine” on admission, one of which was updated to “no known drug allergies” during admission. Medicines were prescribed for 15 patients (19 per cent) before the documentation of allergy, with antimicrobials prescribed for four patients (5 per cent). Three patients were administered potential allergen. A risk-benefit discussion was documented in all cases (Table 1). We were able to obtain written records documenting allergy status for 66 patients before ICU admission and for 46 patients following ICU admission. The allergies documented in the ICIP were in agreement with pre-ICU documentation in 62 of 66 patients’ records (94 per cent) and in agreement with post-ICU documentation in 44 of 46 patients’ records (96 per cent). The ICIP was in agreement with the EPR for 26 of 34 patients (76 per cent) (Table 2) and the written medical record was in agreement with the EPR for 25 of 34 patients (74 per cent).
There was a statistically significant improvement in allergy documentation before prescription of any drug (P=0.01, Table 1) and in agreement between allergy documentation in the written medical record before ICU admission and the ICIP (P=0.01). There was no statistically significant change in the agreement in allergy documentation between the ICIP and the written medical record on ICU discharge (P=0.07). There was no change in the agreement between the EPR and the ICIP (P=0.58) (Table 2).
Discussion
The results demonstrate that the presence of a known allergy was common on admission to ICU with 29 to 39 per cent of patients having a recorded allergy. The preliminary audit demonstrated that despite the significant resources allocated to the ICU, the timely and accurate documentation of allergy was poor with 51.7 per cent of patients not having their allergy documented before administration of a non-emergency medicine (20.7 per cent were antibiotics). Agreement between ICU and pre-ICU records was only 78 per cent. Agreement between ICU and post-ICU records was 81 per cent. Following the implementation of significant process changes there was an improvement in all aspects of allergy documentation.
The proportion of patients who had a non-emergency medicine prescribed before the documentation of allergies reduced to 19 per cent (P<0.01) (5.1 per cent for antimicrobials (P<0.01)). Agreement between the written medical record before ICU and the ICU records also improved significantly to 94 per cent (P=0.01). There was also a clinically significant improvement in allergy agreement between the ICU and post-ICU medical record (81 to 96 per cent) although this did not reach statistical significance.
The lack of change in agreement between the ICU medical record and the EPR was disappointing. However, there was also an unchanging level of agreement between the EPR and the written medical record (initial audit 75 per cent; second audit 74 per cent). This is likely because the completion of the EPR allergy status is not compulsory at any point in a patient’s stay until discharge and this system is used for creation of discharge prescriptions. It is not used for drug prescribing for inpatients and therefore not routinely currently checked at admission.
The audits reported here give an indication of the complexity of systems having a mixture of electronic and paper records. Consequently there is a significant onus upon clinical staff to ensure the accuracy of transcription of information between these different sources, and the potential for error is clearly present. Although an ideal solution would be to change to a single, hospital-wide system of electronic allergy documentation and medication prescribing,[10]
that solution is not presently readily achievable within our institution. To improve the process underpinning allergy documentation a number of changes were implemented following the first audit:
- The ICIP was reconfigured to make the documentation of allergy mandatory within the admission medical clerking
- The medicines reconciliation process was enhanced and prioritised to ensure that the critical care pharmacists confirm allergies with patient’s notes or family doctor
- The documentation of allergy was highlighted as part of the medical trainee induction process into the ICU
The critical care specialty (including the ICU) at GSTT has a specialist critical care pharmacist service six days a week and so allergy confirmation by a pharmacist is achievable within 48 hours of admission for all patients. Although it is acknowledged that the local GP service may not be accessible at weekends, for most patients it is possible to confirm allergy from alternative sources, as discussed previously.
Although difficult to be certain which of these changes had the greatest impact, it is likely to be making the documentation of allergy mandatory within the admission clerking. One of the features within the ICIP is the ability to configure the system to make certain details mandatory. This has meant that the medical notes cannot be saved without allergy being documented — either as a known allergy, as “no known drug allergy” or as “allergies unknown”. We created the field “allergies unknown” to reflect the fact that some patients enter the ICU without significant personal or medical details being known and also to highlight the lack of available information. At this stage the ICIP does not have a medicines library that could prevent known allergens being prescribed; however, the documentation of risk-benefit discussions relating to the prescription of potential allergens uncovered in the second audit give some reassurance that the increased visibility of allergy documentation has been successful. The particular benefit of this systems-wide approach has been to negate the effect of staff turnover and lack of familiarity. Including allergy as a key component of the ICU induction for medical trainees has also helped to address the problem of high staff turnover (over 100 medical trainees annually).
Medicines reconciliation has been shown to be important.[11]
As part of our medicines reconciliation process pharmacists will use a number of relevant sources to ascertain chronic medicines and allergies as well as confirm these with hospital medication charts. This process takes place on admission to and discharge from the ICU and has likely contributed to the improved agreement seen. The formalisation of the role of pharmacists in allergy confirmation further increases the medication safety responsibility of specialist critical care pharmacists within our ICU.
Quality improvement within the ICU has focused on markers such as ventilator-associated pneumonia, central line infections, acquisition of resistant micro-organisms, standardised mortality and lengths of stay.[12],[13]
Many of these markers cover a wide range of interacting factors and hence are useful for examining how the unit as a whole operates throughout a patient’s hospital stay. They also require significant interpretation of a number of interdependent variables and it can be difficult to assess which aspect of care may have greatest impact when considering quality improvement interventions. Unlike established markers of quality, documentation of allergy status is a simple and unambiguous marker to audit.
In the provision of safe patient care within the ICU, medication errors are important.[14],[15]
Much of the focus of safe prescribing has been on individual practice and the provision of appropriate checks within the system. The accurate and timely description and documentation of known allergies and their avoidance should also be considered significant.[16]
There are a number of limitations of this work. The two audits represent a retrospective before-and-after snapshot of allergy documentation over two separate one-month periods 11 months apart. It is not known whether the results are truly representative of the remaining months of the year, or whether the improvements are solely due to the changes implemented, although no other changes occurred relating to allergy documentation. September and October were selected as these are two of the months where there is no junior medical staff changeover, with the aim of making the audit more representative of actual prescribing and documentation practice. Of course it is possible that this may have introduced unrecognised bias. We recognise that the solutions that we propose may not be applicable in all ICUs or hospitals with different systems of prescribing and documentation.
Conclusions
We have described a system-wide approach that, in our circumstances, appears to have contributed to an improvement in safety and a reduction in potential harm through the improvement in allergy documentation. Allergy documentation should be considered a marker for safety and quality within ICU. Solutions to ensuring adequate documentation will be dependent upon local factors, although a system-wide approach involving the multidisciplinary team is most likely to be effective.
About the authors
Cathrine McKenzie, PhD, MRPharmS, is consultant pharmacist for perioperative medicine and critical care, Katie Hatton, MPharm, is senior clinical pharmacist and Nicholas Barrett, FJFICM, is consultant intensivist at St Thomas’ Hospital, Guy’s and St Thomas’ NHS Foundation Trust, London.
Correspondence to: Cathrine McKenzie, Pharmacy Department, St Thomas’ Hospital. Lambeth Palace Road, London SE1 7EH (email catherine.mckenzie@gstt.nhs.uk)
References
[1] Garland A. Improving the ICU: part 1. Chest 2005;127:2151–64.
[2] Harrison DA, Rowan KM. Outcome prediction in critical care: the ICNARC model. Current Opinion in Critical Care 2008;14:506–12.
[3] Bates DW, Cullen DJ, Laird N. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. JAMA 1995;274:29–34.
[4] Solensky R. Hypersensitivity to beta-lactam antibiotic. Clinical Reviews in Allergy and Immunology 2003;24:201–20.
[5] Macy E, Poon K-Y T. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. American Journal of Medicine 2009;122:778.
[6] Borch JE, Andersen KE, Bindslev-Jensen C. The prevalence of suspected and challenge-verified penicillin allergy in a university hospital population. Basic Clinical Pharmacology and Toxicology 2006;98:357–62.
[7] National Patient Safety Agency/National Institute of Clinical Excellence. Technical patient safety solutions for medicines reconciliation on admission of adults to hospital. London: NPSA/NICE, 2007.
[8] O’Dell K. Allergy documentation: strategies for patient safety: Oklahoma Nurse 2006;51:16.
[9] Guys and St. Thomas NHS Foundation Trust. Allergy Policy. London: GSTT, 2010.
[10] Finkelstein JB. E-prescribing first step to improved safety. Journal of the National Cancer Institute 2006;98:1763–5.
[11] Knibbe CA, Tjoeng MM. Clinical pharmacist in intensive care unit saves lives and reduces costs. Critical Care Medicine 2008;36:3184–9.
[12] Garland A: Improving the ICU: part 2. Chest 2005;127:2165–79.
[13] Kahn JM, Fuchs BD. Identifying and implementing quality improvement measures in the intensive care unit. Current Opinion in Critical Care 2007;13:709–13.
[14] Provonsot PJ, Berenholtz SM, Goeschel C, Thom I, Watson SR, Hoyzmueller CG et al. Improving patient safety in intensive care units in Michigan. Journal of Critical Care 2008;23:207–21.
[15] Wilmer A, Louie K, Dodek P, Wong H, Ayas N. Incidence of medication errors and adverse drug events in the ICU: a systematic review. Quality and Safety in Health Care 2010;19:e7 (epub 29 July 2010).
[16] Anon: How to reduce prescribing errors. Lancet 2009; 374: 9706