Abstract
Aim
To identify and prioritise risks associated with medicine shortages in NHS hospitals, and describe how these risks can be managed more safely.
Design
A failure modes and effects analysis (FMEA)
Subjects and settings
The multidisciplinary group involved in managing medicine shortages in a district general hospital.
Results
In total there were 61 failure modes identified and scored for the 14 process steps for managing medicine shortages. The failure mode with the highest criticality score was associated with unmanaged risks when an alternative medicine is supplied in the place of the original medicine that is in short supply. New risks can be introduced when an alternative medicine is supplied for use. These range from the new product not being recognised and not used, being mis-selected for another medicine, or where wrong dose, preparation and administration errors occur. Clinical staff may incorrectly assume the alternative medicine product is to be used in an identical way to the medicine in short supply. A risk assessment checklist was developed for use with alternative medicine products intended for replace medicines shortages. The checklist can be used to authorise alternative medicines for safe use in the hospital and provide a clinical governance audit trail for their introduction.
Conclusion
Although it is often not possible to predict when medicine shortages will occur, risks to patient safety and effective processes for dealing with them and communicating internally and externally can be defined beforehand.
In a review of over 500,000 medication incidents reported to the National Reporting and Learning System between 2005 and 2010, omitted and delayed medicine was the category with the largest number (16 per cent) of reported medication incidents.1 Some of these incidents resulted in severe harm to patients or even death and the National Patient Safety Agency has issued guidance on these risks and how to minimise them.2
There are many reasons why patients are not given their medicines in hospital, including a shortage of products. Medicine shortages arising from manufacturers’ inability to supply products occur frequently in the UK3–6 and elsewhere in the world.7–9 Medicine shortages also occur in hospitals due to failures in stock control and procurement, and a breakdown in the internal supply chain, as well as sudden increases in product demand to meet clinical need.
Patient safety incidents have occurred when medicine doses have been omitted or delayed, and also from the misidentification or misuse of alternative medicine products supplied when there is a shortage of the original medicine. This paper looks at how the risks associated with medicine shortages in NHS hospitals can be managed more safely.
Failure modes and effects analysis
A prospective risk assessment method called failure modes and effects analysis (FMEA) was used to conduct this research. The term “failure mode” refers to the manner in which a failure occurs, while “effects analysis” is the study of the effects of such a failure. FMEA has been widely used in high-risk industries such as aerospace and nuclear power and has started to be used in healthcare.10–13 The tool is designed to promote patient safety by mapping the process of care and identifying failures or shortcomings (failure modes) to understand how and why they occur.
Method
In 2009 the National Patient Safety Agency worked with a multidisciplinary group of staff in the Luton and Dunstable Hospital NHS Foundation Trust to conduct an FMEA to identify risks associated with medicine shortages and to describe methods to manage these risks in NHS hospitals.
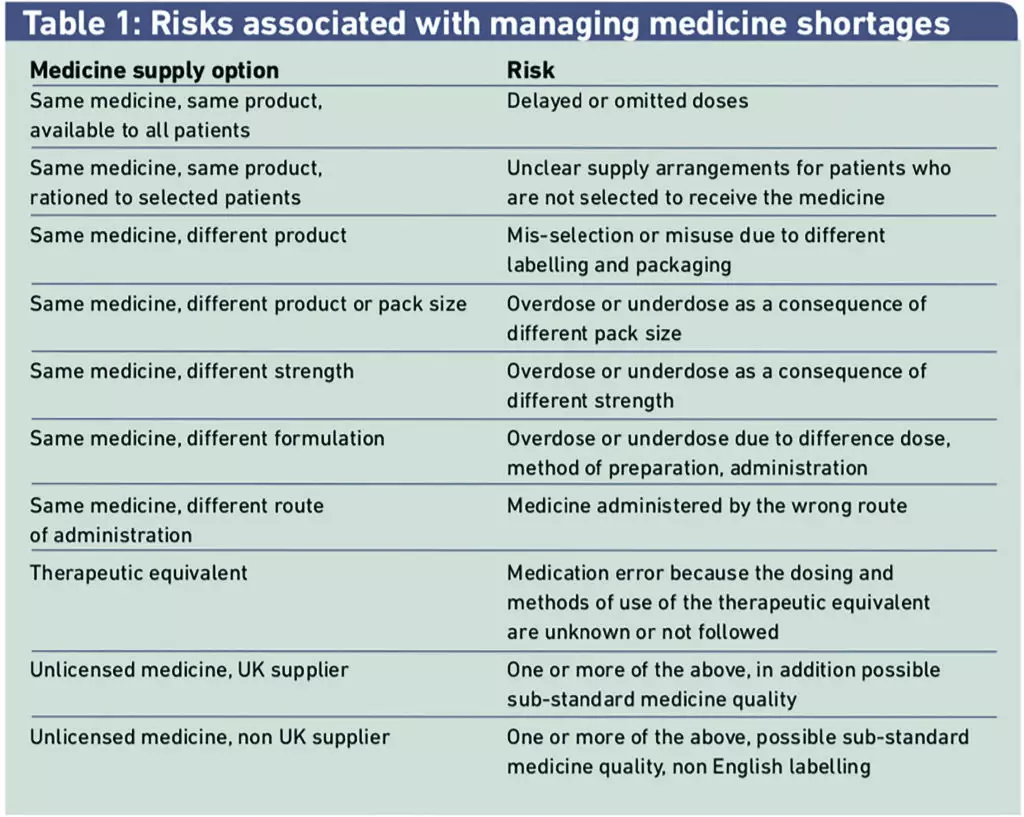
An outline of the generic risks associated with managing medicines shortages was prepared by the NPSA using patient safety incident reports and was shared with the multidisciplinary group at the beginning of the FMEA process. (Table 1)
The FMEA method developed by the Institute for Safe Medication Practices Canada was used.14 The group produced a detailed flow diagram of the steps taken to identify and manage a medicine shortage
in the hospital. The failure modes, their causes and potential clinical outcomes for each step of the process were identified by the group.
Each failure mode was scored for clinical severity on a scale of one to five, with 1 being low and 5 high; frequency of and detectability (values ranging from 1, always, to 4, never). The three scores were then multiplied together to determine a “criticality” score, which has a maximum of 100. The higher the criticality score the more critical the failure mode.
Failure modes were prioritised according to their criticality scores. Additional risk reduction methods were identified for each failure mode.
Results
The process for managing medicine shortages in hospitals
A flow diagram was developed describing 14 steps in managing medicine shortages in hospitals (Figure 1).
Failure modes
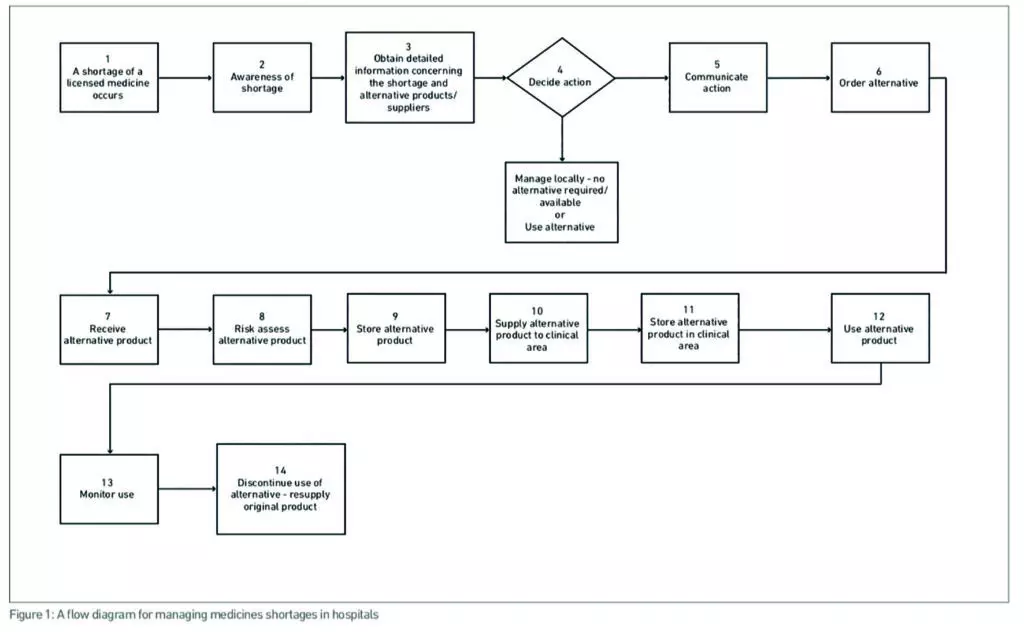
A total of 61 failure modes were identified and scored for the 14 process steps for managing medicine shortages. Failure mode groups with the highest criticality scores and risk reduction methods to minimise these risks are shown in Table 2. Four of the steps in the 14-step process for managing medicine shortages had risks with the highest criticality scores that required the most important actions to minimise risks.
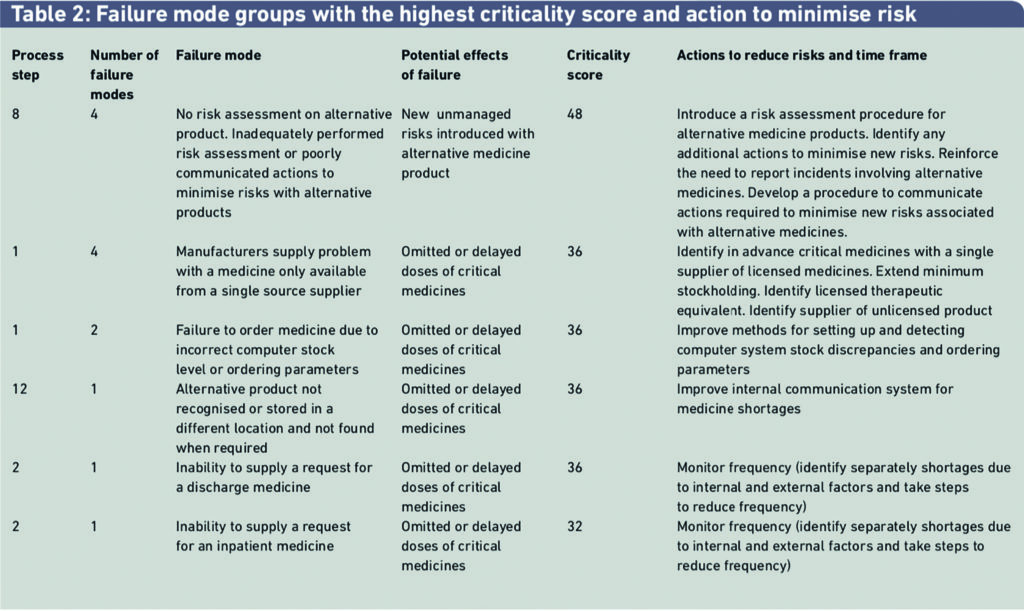
The third example in Table 2 describes a failure mode where the hospital pharmacy fails to order a medicine, with the potential effect that a patient could be harmed if one or more doses were omitted or delayed. The criticality factor was scored as 36 out of a maximum score of 100. Harm from this risk could be minimised by improving methods for monitoring and processing medicine products that need to be ordered. This failure mode is closely linked with another in Table 2, indicating the frequency with which medicines are not supplied on first request and determining the reasons for the failure to supply. Medicine shortages are not always to do with external factors.
Discussion
The failure mode with the highest criticality score was associated with unmanaged risks when an alternative medicine is supplied to replace the medicine that is in short supply.
Results of a recent FMEA study by Macdonald et al at a hospital in the US were similar.15
New risks can be introduced when an alternative medicine is supplied for use. These range from the new product not being recognised and not used, being mis-selected for another medicine, or where dose, preparation or administration errors occur. Clinical staff may incorrectly assume the alternative medicine product is used in an identical way to the medicine in short supply.
Additional risks may occur when the original medicine is reintroduced and the alternative medicine withdrawn from use. New risks are introduced when the alternative medicine is used off-label or is an unlicensed medicine, when the manufacturer’s product information does not include details on how to use the medicine for the intended clinical indication. In some cases unlicensed medicines from outside the UK are not labelled or the product information is not provided in English.
The recommended risk reduction action for new alternative medicine products is to include a formal risk assessment by a senior member of the pharmacy staff. If new risks are identified, action needs to be taken to manage these risks effectively before the alternative medicine is released for use.
An important risk with a high criticality score concerned managing shortages of critical medicines only available from a single manufacturer. Risks could be reduced if these products were identified in advance and contingency plans were put in place to handle potential shortages of them.
Plans should include gathering information about therapeutic alternatives and sources of unlicensed products and deciding whether to increase the trust’s stock of the product. Increases in the amount of stock held need to be considered carefully, taking full account of guidance from the Department of Health and regional procurement specialists. Such increases are contingent on the availability of the product in the supply chain, additional storage space and the impact on the drug budget.
The pharmaceutical market support group and the Department of Health commercial medicines unit have recently produced a list of single-sourced generic products available to manufacturers to encourage other companies to supply these products and reduce the exposure of the NHS to risks arising from medicine shortages.16 Three types of actions were recommended from the FMEA:
- Organisations should ensure that they have put in place strong and effective procedures for managing medicines shortages safely and to mitigate risks associated with replacement products. These procedures need to be subjected to regular review.
- Organisations should review their internal communication systems between pharmacy, nursing and medical staff to ensure they are effective and timely. Where there are financial consequences in managing the medicine shortages internal communication should involve senior financial staff.
- External communication on medicine shortages should be improved at all levels. Hospitals should review their systems to ensure they seek the best information they can obtain to inform their decisions. Manufacturers and distributors and the DoH should be encouraged to improve their communication with NHS hospitals to ensure more timely and accurate provision of information to help manage shortages.
Hospital procedures for managing medicine shortages safely
There are no national guidelines in the UK indicating how medicine shortages should be managed in hospitals and relatively few NHS hospitals have put in place a locally developed formal written procedure. Guidelines exist for the pharmaceutical industry17–18 and there are guidelines for hospitals in the US.19
NHS South West is trialling a regional approach for managing medicines shortages.20 This includes an electronic system to gather and disseminate information across NHS hospitals in the south west with the aim of minimising risk to patients associated with lack of supply. A risk assessment tool provides a traffic-light classification for medicine shortages. The development of national guidelines to help hospitals improve the way they manage risks associated with medicine shortages should be considered a priority.
In the FMEA study in a US hospital conducted by MacDonald et al15 a process for evaluating the potential impact of a medicine shortage on patient safety was described. Safety checklists were developed for use when a same medicine alternative product is required and when a therapeutic equivalent alternative product is required to manage a medicine shortage.
Based on our FMEA we have developed a risk-assessment checklist for alternative medicine products being considered for use to manage a medicine shortage in hospital. This risk-assessment checklist (see Appendix) is intended to be used at step 8 of the process of managing medicine shortages shown in Figure 1. The form clearly documents the risk assessment procedure, identifies the risks of using the alternative medicine and outlines actions and responsibilities to manage these risks. The checklist should be used in conjunction with more detailed guidance on safe labelling, packaging, and quality of licensed21–24 and unlicensed medicines.25 The checklist can be used to provide a formal basis for authorisation of alternative medicines for use in the hospital and a clinical governance audit trail of their introduction.
Improving internal communication on medicines shortages
A national guideline should highlight the importance of hospitals establishing multidisciplinary systems to manage medicine shortages safely. Medical and nursing staff should be formally involved in managing medicine shortages and it should not be seen as the sole responsibility of medicine procurement staff and junior pharmacy staff.
It is desirable that a hospital steering group is established, consisting of a senior doctor, a nurse, a pharmacist and a pharmacy technician, to manage medicine shortages, allocate individual responsibilities and establish an organisational approach to decision-making and communication.
A regular medicine shortage briefing should be prepared for the steering group that identifies where medicine shortages have occurred or are likely to occur and decisions should be recorded on how these shortages are managed and whether new safeguards need to be put in place.
Alongside multidisciplinary oversight of the medicines shortages process it is essential to communicate effectively with clinical users and, where appropriate, patients and carers about medicine shortages. No single method can ensure effective communication. However, written, electronic and verbal methods should be used.
Advising prescribers prospectively of a medicine shortage and use of alternative medicine is challenging and both written and verbal communication by pharmacy staff who visit clinical areas is the best method. Where electronic prescribing systems are in use, electronic notices can be programmed to appear when a medicine in short supply is prescribed, providing details of the shortage and the alternative medicine to be used.
Communicating information about medicine shortages to pharmacy staff, who dispense and supply medicines, and nursing staff, who prepare and administer medicines, should be done orally and in writing. Using small shelf labels in the storage area where the medicine in short supply is kept, together with written information as part of the ward stock supply and possibly bagging or attaching information to the alternative medicine, are simple and effective means of communicating action arising from medicine shortages. Information about medicine shortages can also be communicated via pharmacy dispensing and stock control systems, and via electronic medicine administration systems where they are available.
The regular briefing report on medicine shortages prepared for the multidisciplinary oversight group can be shared more widely and made available on the hospital intranet.
Improving external communications
Gaining information from external sources about the extent and length of the medicine shortage is problematic and information is often incorrect and even misleading. The DoH’s medicines, pharmacy and industry group and commercial medicines unit liaise with industry on a regular basis. However, a lot of the information that is exchanged is deemed commercially sensitive and confidential. The DoH commercial medicines unit circulates briefings on important medicine shortages from time to time.
Until recently, a website set up by University College Hospital London called Solutions (www.uclhsolutions.com) acted as an informal repository of information on medicine shortages in the NHS. The operation of this website has been suspended until further notice. Regional NHS medicines procurement leads also provide proactive and reactive information and advice. These solutions are informal and rely on good quality information from the manufacturers, which is not always available.
In the US, the American Society of Health System Pharmacists publishes a comprehensive medicines shortages resource website (www.ashp.org) and the Food and Drug Administration publishes information about medicine shortages on its website (www.fda.gov). Both sites cover current shortages and resolved shortages and provide information for patients and consumers. Hospitals and others can review and share information about medicines shortages on these sites.
A similar, formally recognised website and system is needed in England and, presumably, in other UK countries. One option is to develop a system based on the University College Hospital London Solutions and the NHS South West initiative.
Pharmaceutical manufacturers and wholesalers seem to have ad hoc systems to communicate with hospitals about medicine shortages. It is usually unclear to customers which department, or individual they should contact to obtain accurate information about medicine shortages. Pharmaceutical manufacturers and wholesalers need to provide a single point of contact on medicine shortage issues for their customers.
Where medicine shortages and use of alternative medicines have an impact on medicine use in the community either via discharge medicines or outpatient medicines, communication with primary care professionals is needed. Communicating information to patients and carers verbally, together with written information for them to share with primary care staff is one method. In addition, direct written communication with primary care staff, when their patients are supplied alternative medicines in response to a medicine shortage will help minimise risks arising in primary care.
Conclusion
The FMEA identified the failures in managing medicines shortages in hospitals that are most likely to be associated with serious patient safety incidents. Although often it is not possible to predict when medicine shortages will occur, effective processes for dealing with them and communicating internally and externally can be defined beforehand. We have described actions that NHS hospitals and others can take to improve the way they manage risks to patient safety arising from medicine shortages.
About the authors
David Cousins is associate director, safe medication practice and medical devices at the NHS Commissioning Board Authority, and was previously head of patient safety, safe medication practice and medical devices at the National Patient Safety Agency.
Mary Evans is chief pharmacist at Luton and Dunstable NHS Foundation Trust.
Kevan Wind is medicines procurement specialist pharmacist, NHS London and East of England
Correspondence to: David Cousins (email dcousins@nhs.net); Mary Evans (email Mary.Evans@ldh.nhs.uk), or Kevan Wind (email Kevan.Wind@southend.nhs.uk)
References
- Cousins D, Gerrett D, Warner B. A review of medication incidents reported to the National Reporting and Learning System in England and Wales over six years (2005–10). British Journal of Clinical Pharmacology 2012 (in press).
- National Patient Safety Agency. Rapid response report. Reducing harm from omitted and delayed medicines. London: NPSA; 2010.
- Barron K. Medicine shortages are not improving and it is time to find a practical solution. Pharmaceutical Journal 2011;287:680.
- Taylor L. MPs to probe prescription drug shortages. Pharma Times Online 22 November 2011. Available at: www.pharmatimes.com/ Article/11-11-22/MPs_to_probe_prescription_ drug_shortages.aspx (accessed 28 June 2012).
- Donnelly L. Drug shortages cause delays for cancer patients. Daily Telegraph 6 February 2011. Available at: www.telegraph.co.uk/ health/healthnews/8305646/Drug-shortages-cause-delays-for-cancer-patients.html (accessed 28 June 2012).
- Karr A. Where have all the medicines gone? Pharmaceutical Journal 2001;267:197–8.
- Chabner BA. Drug shortages. A critical challenge for the generic drug market. New England Journal of Medicine 2011;365:2147–9.
- Institute for Safe Medication Practices. Medication Safety Alert. 29 July 2010. Available at: www.ismp.org/newsletters/acutecare/ articles/20100729.asp (accessed 28 June 2012).
- Baumer AM, Clark AM, Witmer DR et al. National survey of the impact of drug shortages in acute care hospitals. American Journal of Health-System Pharmacy 2004;61:2015–22.
- Senders JW, Senders SJ. An introduction to failure modes and effects analysis in medicine. In: Cohen MR (ed). Medication errors: 2nd edition. Washington, DC American Pharmacists Association 2006.
- Smetzer JL, Cohen MR. An application of failure modes and effects analysis In: Cohen MR (eds) Medication errors: 2nd edition. Washington, DC American Pharmacists Association 2006.
- Williamson S, Wake N, Donovan G. FMEA: a new approach to manage high risk medicines. British Journal of Clinical Pharmacy 2009;1:329–32.
- Shebl N, Franklin B, Barber N, Burnett S, Parand A. Failure mode and effects analysis: views of hospital staff in the UK. Journal of Health Services Research and Policy 2011;17:1–7.
- Institute for Safe Medication Practices, Canada. Canadian Failure Modes and Effects Analysis Framework.2007. Available at: www.ismp-canada.org/fmea.htm (accessed 28 June 2012).
- Macdonald EA, Fox ER, Tyler LS. Drugshortages: Process for evaluating impact on patient safety. Hospital Pharmacist 2011;46:943–51.
- Department of Health, Commercial Medicines Unit. Injectable medicines with a single source of supply. 2011. Available at: http://cmu.dh.gov.uk/files/2011/11/111010-Products-with-Single-Source-on-contract-PDF. pdf (accessed 28 June 2012).
- Department of Health and The Association of British Pharmaceutical industries. Notification and management of medicines shortages. Best practice guidelines 2007. Available at: www.dh.gov.uk.
- Department of Health and British Generic Manufacturing Association Notification and management of medicines shortages. Best practice guidelines 2007. Available at: www.dh.gov.uk.
- Fox ER, Birt A, James KB, et al. Guidelines on managing drug product shortages in hospitals and health systems. American Journal of Health-System Pharmacy. 2009;66:1399–406.
- NHS South-West. South West Medicines Safety Partnership. NHS South West/Association of the British Pharmaceutical Industry Medicines Safety Partnership. Medicines shortages. Improving patient safety through effective management of medicines shortages.2009. Available at: www.swmit.nhs.uk/SWMSP_ MedShortages.htm (accessed 28 June 2012).
- National Patient Safety Agency. Design for patient safety. A guide to the graphic design of medication packaging. 2nd ed. 2006.Available at: www.nrls.npsa.nhs.uk/resources/ collections/design-for-patient-safety/ ?entryid45=63053 (accessed 28 June 2012).
- National Patient Safety Agency. Design for patient safety. Labelling and packaging guidelines for injectable medicines. 2008. Available at: www.nrls.npsa.nhs.uk/ resources/?entryid45=59831 (accessed 28 June 2012).
- NHS Pharmaceutical Quality Assurance Committee. Quality assurance and risk assessment of licensed medicines for the NHS. Version 1. 2004. Available at: www.qcnw. nhs.uk/docs/QA & Risk Assessment of licensed Medicines for the NHS.pdf (accessed 28 June 2012)
- Medicine and Healthcare products Regulatory Agency. Best practice guidance on labelling and packaging medicines. Guidance Note 25. 2003. Available at: www.mhra.gov.uk/home/ groups/comms-ic/documents/publication/ con007554.pdf (accessed 28 June 2012)
- NHS Pharmaceutical Quality Assurance Committee. Guidance for the purchase and supply of unlicensed medicinal products; notes for prescribers and pharmacists. 3d ed. 2004. Available at: www.nelm.nhs.uk.