Abstract
Aim
To identify whether a proactive clinical pharmacy review of a patient’s treatment at the point of admission could potentially avoid adverse events.
Design
A multicentre prospective study.
Subjects and setting
All acute medical patients admitted over a five-day period to seven acute NHS trusts in England and Wales.
Outcome measures
Type, severity and outcome of pharmacist interventions.
Results
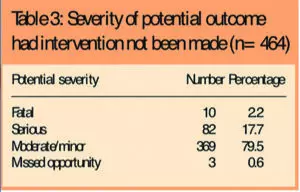
Pharmacists reviewed 791 of 1,170 medical patients. 464 pharmacist contributions were made in 298 patients, 391 (84%) of which were accepted by the relevant medical staff. One was rejected and 72 (16%) were unresolved at the time of reporting. 82 (17.7%) were considered to have prevented potentially serious events. The most common intervention (41.4%) was to add a drug that had inadvertently been omitted from the prescription.
Conclusion
There is a role for a proactive pharmacist review of medical patients on admission to identify the medication needs of individual patients and to correct prescriptions where these are not in line with those needs.
Medication errors are a significant cause of patient morbidity and mortality. In the UK, they account for approximately 20 per cent of all iatrogenic disease.1 A recent international systematic review highlighted that medication errors on admission affected up to 67 per cent of patients and were potentially harmful.2 Since many of the medicines that patients take during their hospital stay are a continuation of existing treatment, it is important that medication histories established on admission are thorough, accurate, reviewed appropriately, and subsequently translated into a safe and precise inpatient prescription.3 Although clinical pharmacy review has been shown to highlight and correct medication-related errors4 the retrospective nature of prescription review leads to significant delays in the correction of errors,5 potentially allowing erroneous drug treatment to be administered to patients.
This is the first multi-centre study that has set out to identify whether a proactive clinical pharmacy review of a patient’s treatment at the point of admission could potentially avoid adverse events.
Method
Clinical pharmacists in seven different hospital trusts were asked to review all acute medical patients (including care of the elderly) as close to the time of admission as possible during a single week (Monday to Friday) in October 2002. They were asked to record details of any pharmacist contribution made as part of “normal care” using a standardised reporting form adapted from the system used by Strand and colleagues in Minnesota.6 The number of patients seen by a pharmacist, including those without contributions, was recorded as was the total number of medical admissions per day. The number of admissions was obtained using local trust information systems. The sites selected were hospitals known to be providing review of individual patient’s need for medication as soon as possible following admission.
This study involved a review of the patient’s medication history and an assessment of a patient’s need for a medicine with the identification of the most appropriate medicine to meet that need, as early as possible after admission. The outcome of this assessment was then compared with the existing prescription. Any anomalies identified were then discussed with the prescriber.
The assessment made by the pharmacists was informed by a medication history from the patient or carer and an examination of the medicines brought into hospital, together with any documentation accompanying the patient. Details of the admitting physician’s assessment, including an identification of diagnosis made and any co-morbidity recorded, were also reviewed. Where necessary, clarification of the details of the drug history was sought direct from the primary care physician or the community pharmacy.
Data collected included details of the reason for intervention (see Panel 1), the outcome of the intervention in terms of physician acceptance, the main drug(s) involved and a detailed description of the problem identified.
Panel 1: Intervention categories
- Need for additional drug Pharmacist assessment identified that the patient needed a drug that was not currently prescribed*
- Unnecessary drug therapy Prescription contained items that patient assessment identified as no longer required*
- Wrong drug Prescription contained items that were not indicated for the individual patient (includes some transcription of other peoples’ prescription)*
- Dose too low The dose prescribed was lower than usually taken y the patient or was lower than the assessed patient need*
- Adverse drug reaction Prescription included drug for which the patient demonstrated an adverse drug reaction
- Dose too high The dose prescribed was higher than usually taken by the patient or was in excess of the assessed patient need*
- Compliance Patient was not taking medicines as directed by the physician before admission (excludes interventions listed under other categories)
- Miscellaneous Interventions that could not otherwise be classified, eg, incomplete prescriptions or lack of dose schedules
*Validation of these intervention categories is provided by physician acceptance of the interventions
An assessment of the potential clinical significance of the intervention was made at the time by the pharmacists involved and subsequently reviewed by a multidisciplinary panel consisting of two clinical pharmacists (AS, DH) and a clinical pharmacologist (SC) working independently. Variations were resolved by discussion. The potential outcome is identified as what would have been likely to occur had no intervention been made.
Data were collated and analysed using an MS Access database. Data were then reviewed by three of the researchers DH,AS and SC to ensure standardisation and consistency of categorisation and scoring.
Guidance from the lead centre’s ethics committee was that ethics committee approval was not required.
Results
A total of 1,170 patients were admitted during the study period, of whom 791 (72 per cent) were seen by a pharmacist on admission. A breakdown of the type of intervention made is summarised by hospital in Table 1.
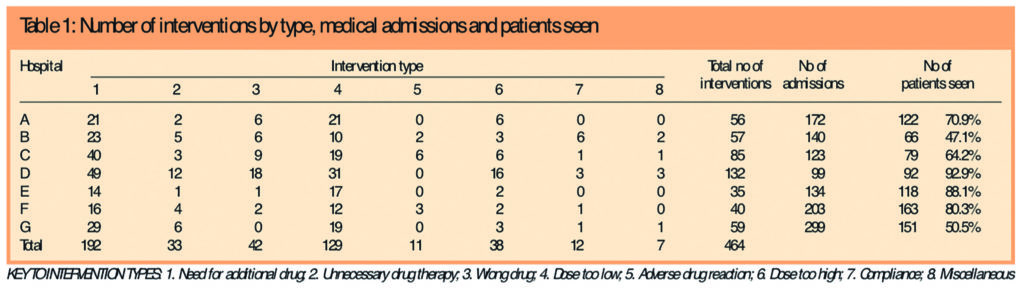
A total of 464 interventions were recorded with an average of 1.5 interventions per patient (in the 38 per cent of patients who required an intervention). Three hundred and ninety-one interventions (84 per cent) were accepted by the medical staff. One was not accepted (a recommendation to change co-proxamol due to an interaction with warfarin, leading to a high international normalised ratio was rejected because the doctor decided to monitor this closely instead).
A further 72 (16 per cent) were unresolved at the time of reporting — these interventions had been communicated to the prescriber but a response had not been received within the data collection period (no attempt was made retrospectively to pursue or clarify the outcome of these interventions).
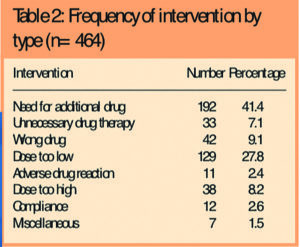
Table 2 illustrates the frequency of the different types of intervention made.
The potential severity of the outcome, had the interventions not been made, is shown in Table 3 as well as three opportunities that were missed potentially to improve patients’ treatment.
Examples of potentially fatal or serious errors identified included:
- Digoxin 1mg prescribed instead of doxazosin 1mg
- Patient with acute coronary syndrome prescribed sumatriptan (ischaemic heart disease being a contraindication to sumatriptan therapy)
- Levothyroxine 250mcg prescribed instead of 25mcg
- Four incidents of antibiotics being prescribed for patients with a documented hypersensitivity to that antibiotic
- Azathioprine prescribed in error, ie, no indication for the prescription
Examples of omitted drugs included three cases of anticonvulsants, two cases of regular cardiac medication and three cases of insulin or oral antihyperglycaemic therapy. (These represent drugs prescribed for chronic conditions that, when omitted, may have been associated with avoidable adverse events including seizures, angina or hyperglycaemia, respectively.)
Discussion
The scale of prescription errors in the UK is poorly defined. A retrospective
review of prescriptions by pharmacists in London identified a prescribing error rate of 1.5 per cent with approximately one third of these identified as
serious.7 In their summary of studies examining medication histories on admission,Tam and colleagues found that up to 67 per cent of histories contained some error with between 10 and 61 per cent containing an omission error (defined as the deletion of a drug used before admission).2
In this study, acts of omission were also identified as the most common intervention (41 per cent of interventions). The acceptance of these interventions by medical staff suggests such omissions were not made deliberately in response to patients’ changing medical conditions, although poor documentation of such decisions is a confounding factor. Although a number of the omissions had low clinical significance some had the potential for serious consequences, such as failure to prescribe anticonvulsants for patients with epilepsy or insulin for patients with diabetes. Although this need not necessarily be life-threatening or result in drug toxicity, it has serious implications for efficacy, and hence morbidity and length of hospital stay. Other prescription errors included under-dosing of patients or, more commonly, failure to specify a dose (28 per cent) and the prescribing of excessive doses (8 per cent). We also found 42 prescriptions for the wrong drug prescribed in error (9 per cent), eg, digoxin rather than doxazosin.
Assigning a severity score to interventions is subjective and since, by their nature, interventions change the potential outcome of a prescription error, the potential outcomes (had there been no intervention) are difficult to identify. Other retrospective case review studies have examined actual outcomes observed. The first study identified a medication error rate of 5.3 errors per 100 orders or 1.4 errors per admission, although only a minority was associated with an injury to the patient.8 In a further study a rate of 6.5 actual and 5.5 potential adverse drug events per 100 admissions were found,9 1 per cent of which were fatal and 12 per cent of which had life-threatening outcomes. Although the severity rates reported in these studies are higher, the latter study only examined outcomes from the actual (not the potential) adverse drug events. These studies do, however, highlight the potential for a medication error to translate into real harm for patients. This potential for medication errors should be set in the context of the potential for the drugs themselves to cause harm. Adverse drug reactions, many of which were avoidable, have been shown to cause significant morbidity and mortality, accounting for 6.5 per cent of hospital admissions and 0.15 per cent of deaths.10
One difference between this study and other pharmacist intervention studies was the emphasis on promoting “optimal” care. In line with Strand’s recommendations,6 pharmacists identified the patient’s need for a medicine and did not simply focus on whether the dose was appropriate or whether there were potential drug interactions, etc. We found that 33 interventions (7 per cent) identified an unnecessary drug, eg, lansoprazole discontinued four months previously. This exposes patients to the risk of adverse events (including adverse drug reactions) from agents that have no therapeutic benefit for that individual.
The causes of the errors identified are not discernible from this study. Possible explanations include poor communication from primary care and the admitting doctor being focused on the admitting condition and less concerned about treatment of co-morbidities that were unrelated to the admission. The perceived relative unimportance of prescribing and the doctor being “busy” at the time of prescribing have been highlighted elsewhere11 as contributing factors to prescribing errors.
We have demonstrated that prescribing errors can be identified and corrected by a pharmacist proactively reviewing patients on admission. As a key member of the multidisciplinary team, working alongside medical staff, a pharmacist can ensure that treatment is adjusted to meet individual patients’ needs in light of all the information about the patient and the drug. The potentially serious nature of some of the errors made underlines the need for a timely review by a pharmacist if we are to avoid patient harm as a consequence of error.
The study has a number of potential weaknesses. Although a standardised process was followed for intervention recording and rating, which identified a high level of correlation between centres in the type of interventions made, it was not possible to standardise the training and experience of the pharmacists involved in the study in the different centres. This presents a potential for variation in performance between the different sites.
In addition the assessment of clinical severity was subjectively made by the pharmacist at the scene and was then reviewed later from abridged information provided to the reviewers. This lends a level of subjectivity to the clinical significance rating leading a number of interventions to be classified as moderate or minor.
Conclusion
There is a role for a proactive pharmacist review of medical patients on admission, to identify the medication needs of individual patients and to correct prescriptions where these are not in line with these needs. This is a consistent finding across all hospitals included in the study and is not unique to a particular hospital. This study adds weight to the recommendations of the Audit Commission that reviewing medication needs on admission should be a major focus for pharmacy services.12
Acknowledgements
Many thanks to all the pharmacists that collected data for this study and made it happen. Particular thanks are due to Sue Ashwell, Mike Pollard, Ffion Johnstone, Dave Thornton and Andrew Barker.
This paper was accepted for publication on 10 November 2006.
About the authors
Ann Slee, MSc, MRPharmS, is clinical lecturer at the School of Pharmacy and Chemistry, Liverpool John Moore’s University, and clinical lead, ePrescribing Programme, NHS Connecting for Health. At the time of the study she was director of pharmacy at Conwy and Denbighshire Trust.
Keith Farrar, MPharm, MRPharmS, is clinical lecturer at the School of Pharmacy and Chemistry, Liverpool John Moore’s University. At the time of the study he was director of pharmacy at Wirral Hospitals NHS Trust.
Don Hughes, MSc, MRPharmS, is director of pharmacy at Conwy and Denbigh NHS Trust. At the time of the study he was director of pharmacy at the Countess of Chester Trust.
Simon Constable, MB BS, MRCP(UK), is lecturer in clinical pharmacology in the department of pharmacology and therapeutics, University of Liverpool.
Correspondence to: Ann Slee (e-mail ann.slee@nhs.uk)
References
- Department of Health. An organisation with a memory. London: HM Stationery Office; 2000.
- Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ 2005;173:510–15.
- McFadzean E, Isles C, Moffat J, Norrie J, Stewart D. Is there a role for a prescribing pharmacist in preventing prescribing errors in a medical admissions unit? Pharmaceutical Journal 2003;270:896–9.
- Hawkey CJ, Hodgson S, Norman A, Daneshmend TK, Garner ST. Effect of reactive pharmacy intervention on the quality of hospital prescribing. BMJ 1990;300:986–90.
- Farrar KT, Stoddart MJ, Slee AL. Clinical pharmacy and reactive prescription review — time for a change? Pharmaceutical Journal 1998;260:759–61.
- Cipolle RJ, Strand LM, Morley PC. Pharmaceutical care practice. New York: McGraw-Hill; 1998.
- Dean B, Schachter M, Vincent C, Barber N. Prescribing errors in hospital inpatients: their incidence and clinical significance. Quality and Safety in Health Care 2002;11:340–4.
- Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape LL. Relationship between medication errors and adverse drug events. Journal of General Internal Medicine 1995;10:199–205.
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events. JAMA 1995;274:29–34.
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ 2004;329:15–19.
- Dean B, Schachter M, Vincent C, Barber N. Causes of prescribing errors in hospital inpatients: a prospective study. Lancet 2002;359:1373–8.
- Audit Commission. A spoonful of sugar, medicines management in NHS hospitals. London: Audit Commission; 2001.
You may also be interested in
