Key points:
- The number of older people living with HIV (PLWH) is increasing: currently one in three of those accessing care in the UK are >50 years old; they are expected to become the majority age group during the next decade.
- PLWH have a higher incidence of comorbidities, including those not directly associated with HIV infection, which commonly appear about ten years earlier than in the general population. This results from a combination of factors, including accelerated and accentuated ageing and higher rates of some behavioural risks (e.g. smoking), which should be addressed where possible.
- Multimorbidity, frailty and polypharmacy occur at higher rates and younger ages in PLWH. This is associated with significant risks of drug–drug interactions, including with non-prescription products.
- These factors may impact negatively on adherence to antiretroviral therapy and other treatments, which could jeopardise some of the recent gains in life expectancy.
- Medicines optimisation for older PLWH should be a priority for pharmacists and the wider healthcare team. Robust systems should be established to facilitate clear communication between providers, and a full medication review should be conducted, ideally by an HIV specialist pharmacist, at least annually.
- Pharmacists in all sectors who encounter PLWH should be mindful of the medicines optimisation issues associated with HIV and ageing.
- Stigma, even in healthcare settings, remains prevalent and is a significant barrier to normalising HIV as a medical condition.
- New models of care need to be developed and evaluated to address the evolving context of HIV as we approach the end of the fourth decade since the epidemic began.
In 1981, the first manifestations of what subsequently became known as the Acquired Immune Deficiency Syndrome (AIDS) were reported in the United States[1]
. The cause was unknown; there was no test and no treatment. As the number of cases grew, it became clear that in resource-rich countries (including the UK), AIDS was disproportionately affecting men who have sex with men (MSM)[2]
. Societal norms and legislation were very different at this time, and stigma and discrimination were commonly experienced by MSM, both in relation to their sexuality and to HIV, even in healthcare settings[3]
.
By the mid-1990s, HIV had been identified as the cause of AIDS[4]
, antibodies to the virus could be detected by a blood test[5]
and combination antiretroviral therapy (ART) was beginning to be used[6]
. This was followed by the development of ‘viral load’ testing (HIV RNA measurement), which allowed the effectiveness of therapy to be monitored[7]
, and genotyping, which enabled treatment selection to be assisted by the detection of resistance mutations[8]
. In 1996, within months of ART being introduced, the so-called ‘Lazarus effect’ began to be reported[9]
: where patients who were dying of AIDS who underwent ART became people living with HIV (PLWH). The death rate began to fall dramatically; however, for this first generation of ART beneficiaries, life expectancy remained uncertain, as there was no precedent to gauge how long the treatments would work. Today, HIV infection in the UK is no longer an untreatable and inevitably fatal condition; it can usually be controlled with appropriate treatment[6]
.
Early ART regimens were expensive and complex, generally involving at least ten pills per day, divided into two to four dose times, often with specific dietary or timing requirements. Consequently, it was challenging for people to sustain the high level of adherence (>95% doses taken correctly) that is required to achieve a durable therapeutic response (continued suppression of HIV with no emergent resistance)[10]
. Viral resistance development restricts future treatment options, which was particularly crucial at the time because there were a limited number of alternatives[11]
. Despite this, ART was (and still is) one of the most cost-effective, life-changing and life-saving healthcare interventions[12]
. Subsequent therapeutic developments have resulted in simpler, more effective treatments, and the mortality rate continues to fall[13]
. Recent UK figures show that 613 people with HIV died in 2015, but more than half of these deaths were attributable to other causes[14]
.
As a result, the average age of people accessing HIV care in the UK is increasing: 45 years in 2015 compared with 39 in 2006 (Figure 1)[14]
. This trend is set to continue with life expectancy increasing in the general population[15]
and in those living with HIV[16]
. Most PLWH who are diagnosed soon after acquisition of the virus and who start ART immediately can now expect to live a similar lifespan to their counterparts without HIV. The increase in life expectancy in PLWH has been driven by the rising uptake of effective ART, and evidence-based treatment guidelines from The British HIV Association (BHIVA) and the World Health Organization, which now recommend early ART initiation (as soon after diagnosis as possible, depending on the patient’s readiness)[17],[18]
. In 2015, 96% of patients accessing UK HIV care were already receiving ART, and 94% of those on ART had a suppressed viral load (HIV RNA <200 copies/ml)[14]
.
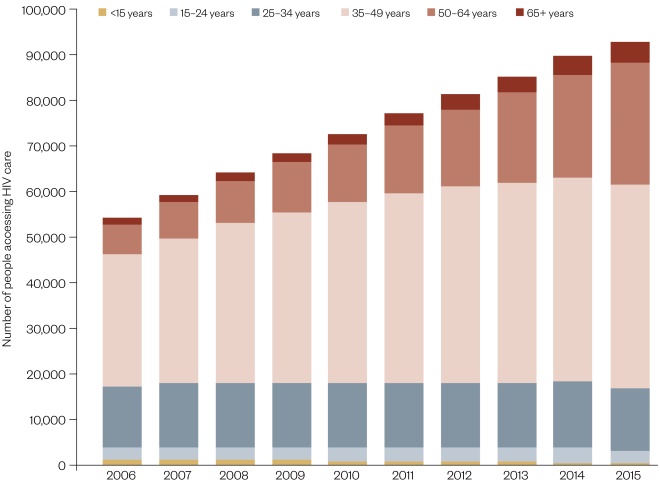
Figure 1: People diagnosed with HIV accessing HIV specialist care, by age group: UK, 2006–2015
Source: Public Health England. HIV in the UK, 2016 report
This review discusses recent literature on ageing and HIV, and implications for optimising the health and wellbeing of older PLWH in the UK. It includes issues affecting people who have been diagnosed with HIV later in life, as well as older people who have been living with HIV for many years. Some areas covered also affect other patient groups, and/or younger PLWH, but arguably have greater relevance to those who are older. These include: multimorbidity, polypharmacy, adherence, drug–drug interactions (DDIs), medicines optimisation and models of care. The description and management of specific comorbidities is not discussed: for this, the reader is referred to the recent reviews by Serrano-Villar et al.
[19]
, and Sangarlangkarn and Appelbaum[20]
.
Older people living with HIV
When considering the definition of ‘older people’, several factors are pertinent, including chronological age, biological age (or functional abilities) and social roles, and it is well recognised that these are not synonymous[21]
. There is no universal definition, and a variety of criteria have been used in different circumstances. While recognising that chronological age may not be the best prognostic indicator[22]
, for the purposes of this review, an ‘older person’ is someone aged 50 years or more, a figure that is now commonly used in the context of HIV[23],[24]
. However, this definition encompasses a huge age range, including PLWH in their 90s. For this reason, more specific descriptors (e.g. ‘young-old’, ‘old-old’ and ‘oldest-old’) may sometimes be used to identify those with particular characteristics or requirements within the spectrum. Unfortunately, there is no consistent definition for each of these subgroups[25],[26]
.
Broadly speaking, there are two groups of older PLWH: those who were diagnosed before the age of 50 years, and have been living with the virus and taking ART for a longer time (the ‘ageing’ group); and those who were diagnosed at an older age (the ‘aged’ group)[27]
. Both groups are increasing in number and as a proportion of the whole population with HIV. In the ‘ageing’ cohort, one in three of those engaged in HIV care in the UK are currently aged 50 years or over (one in four of whom are women), compared with one in seven a decade ago[28]
; this is projected to increase to more than half of PLWH by 2028[29]
. The increase in the ‘aged’ group has been similarly demonstrated: one in six people diagnosed in 2015 were aged 50 years or over (one in four of whom were women), compared with 1 in 11 in 2006[28]
.
Optimising health by reducing late diagnosis
In high-income settings, such as the UK, the life expectancy of people who are diagnosed soon after infection with HIV, start ART before significant immunosuppression (i.e. with a CD4 T-lymphocyte count of >500 cells/mm3) and remain on treatment with a fully suppressed viral load, is now approaching that of the general population[30]
.
From 2006 to 2015 the estimated number and proportion of people living with undiagnosed HIV decreased considerably from 21,600 (31%) to 13,500 (13%)[31]
. However, despite a corresponding steady decline in late diagnoses (people with a baseline CD4 count of <350 cells/mm3), older PLWH are still significantly more likely to be diagnosed late; almost 60% of those aged over 50 years compared with around one in three younger people[28],[32]
. Reducing the number of people who are unaware of their HIV infection remains a high priority, and is an important element of any strategy to optimise the health and wellbeing of older PLWH[33]
. Early diagnosis is also vital in reducing onward transmission of HIV[14]
.
Older people may be more at risk of acquiring HIV, or remaining undiagnosed, for a variety of reasons[27]
, including:
- Older people are less likely to require contraception and may not perceive themselves to be at risk of sexually transmitted infections, so may be less likely to use male or female condoms;
- Condom use can be problematic for men with erectile dysfunction, which is more common in older men;
- Post-menopausal women may be more susceptible to acquiring HIV, partly due to vaginal dryness and increased fragility of the vaginal epithelium;
- Symptoms of recent HIV infection (e.g. weight loss, fatigue) may be dismissed simply as signs of ageing;
- Healthcare professionals appear to be reticent to ask older people about their sexual behaviour or to offer an HIV test[27]
.
The three demographic groups who are most represented among PLWH in the UK are MSM, black Africans (particularly women, including those born in the UK) and white heterosexuals[28]
. PLWH who are in a low-HIV prevalence demographic group (e.g. older white heterosexuals who have never injected drugs) may be less likely to be offered a test when presenting with HIV indicator symptoms, which could contribute to the consistently high rates of late diagnosis[34]
.
Specific issues for long-term survivors
Many of the longest-term survivors (from the pre-ART era) continue to be significantly impacted by the legacy of the early years of the epidemic; many have suffered multiple HIV-related bereavements, and ‘survivor guilt’ and post-traumatic stress disorder (PTSD) have been documented[35]
, some of which are anecdotal[36]
, and it has been argued that complex post-traumatic stress, or a distinct AIDS survivor syndrome are a more accurate diagnosis than PTSD. Living for many years with an uncertain prognosis, financial insecurity, a lack of caregivers, and historical and ongoing stigma and discrimination are some of the factors that contribute to a high burden of depression and anxiety in this patient cohort[26]
. Older PLWH who have had a long history of involvement with the epidemic may be more likely to require additional support to successfully manage the challenges of ageing with HIV[37],[38]
.
Manifestations of ageing in older people living with HIV
Some conditions that commonly occur in older people in the general population are found more frequently and at an earlier age in PLWH[39]
. These HIV-associated non-AIDS (HANA) conditions include certain malignancies, neurocognitive degeneration, fragility fractures, and cardiovascular, hepatic and renal diseases[19],[20]
.
Aetiology of ageing in people living with HIV
Both HIV infection and ageing are characterised by:
- Chronic inflammation because of the production of pro-inflammatory cytokines (sometimes termed ‘inflammaging’);
- Ongoing immune activation;
- Immunosenescence (the immunological changes that accompany ageing)[40],[41]
.
HIV and ageing are both also associated with an increased incidence of frailty[22],[42]
(See ‘Box 1: What is frailty?’)[43]
. Identifying and understanding the aetiology of HANA conditions and the inter-relationship between HIV and ageing is vital if we are to develop interventions to address the underlying mechanisms and thus improve the health outcomes of older PLWH.
Box 1: What is frailty?
Frailty:
- Is a clinically recognised state of increased vulnerability;
- Is associated with the ageing process;
- Is characterised by a decline in the body’s in-built physical and psychological reserves;
- Increases the risk that an apparently minor event will precipitate a dramatic decline in physical or mental wellbeing;
- Varies in severity and can improve or deteriorate over time.
Source: British Geriatrics Society, Royal College of General Practitioners and Age UK.
Fit for frailty: consensus best practice guidance for the care of older people living with frailty in community and outpatient settings. British Geriatrics Society 2014. Available from: http://www.bgs.org.uk/index.php/resources-6/bgscampaigns/fit-for-frailty
There has been much debate about whether the increased incidence of comorbidities in PLWH at younger ages reflects an accelerated or accentuated ageing process[44]
, or if the differences can be explained by higher rates of behavioural and lifestyle risk factors (e.g. cigarette smoking) and the lack of appropriate control groups in studies[45]
(see ‘Box 2: definitions of accelerated and accentuated ageing’).
Box 2: Definitions of accelerated and accentuated ageing
Accelerated ageing:
- HIV induces pathways and mechanisms that are part of the ageing process;
- Geriatric syndromes occur at a significantly earlier age than in matched HIV-negative controls.
Accentuated ageing:
- HIV is an additional risk factor for specific comorbidities;
- Prevalence of these comorbidities is higher at all ages in matched HIV-negative controls.
There is now considerable evidence from epigenetic studies to support the existence of accelerated ageing as a contributory factor[46]
; for example, a decrease in DNA methylation associated with HIV infection has been shown to predict a lower CD4:CD8 T-lymphocyte ratio, which is associated with ageing and disease progression[47],[48]
. It has also been mooted that infection with cytomegalovirus (CMV) may play a role in accelerated immune ageing, in both those with and without HIV. However, accentuated ageing may also play a role in some comorbidities, including diabetes, and cardiovascular and renal disease, which are more prevalent in PLWH at all ages[20],[44]
.
Differences in morbidity rates between PLWH and HIV-negative controls are frequently less significant once the study populations are appropriately matched for known risk factors for a condition, not just for age, sex and ethnicity; for example, controlling for smoking, alcohol, low body mass index, socioeconomic status and concomitant medications when assessing low bone mineral density and fracture risk[45]
. However, some differences remain: for example, several studies have shown that smoking is more strongly associated with cardiovascular disease and bacterial pneumonia in PLWH, reinforcing the importance of encouraging smoking cessation[49]
. Conversely, some lifestyle changes may reap greater rewards in PLWH (e.g. the benefits of moderate exercise on cognitive function and general health)[50]
.
Treatment toxicities have also been implicated in the ageing process[51]
, including the development of cardiovascular, renal and hepatic disease; however, the incidence of these conditions has been shown to be higher in people with uncontrolled HIV compared with those on effective treatment, therefore, ART is certainly not the sole or major cause[52]
. Similarly, nucleoside/nucleotide reverse transcriptase inhibitors have been proposed as a possible mechanism for accelerated cell senescence; in vitro they inhibit telomerase, resulting in shortened telomeres, which are found in ageing cells[46]
. However, in vivo these drugs undergo intracellular phosphorylation and are used in combination with other antiretrovirals, and it is not possible to confidently deduce the clinical impact from in vitro studies[51]
. The Data collection on Adverse events of Anti-HIV Drugs (D:A:D) study group has been collecting data on ART toxicity from multiple large cohorts since 1999[53]
. Their findings have informed treatment guideline changes, including recommendations for ART choice in older PLWH and/or those at higher risk of specific toxicities. Recently licensed antiretrovirals appear to be associated with a lower incidence of toxicity, and treatment can now be tailored to take into consideration individual cardiovascular, renal, hepatic and bone density risk factors.
Multimorbidity and frailty
Multimorbidity is extremely common in PLWH (as detailed below). The number of HANA comorbidities increases over time and typically occur ten years earlier than in those who are HIV negative[54],[55]
. For example, in the Swiss HIV Cohort Study, just over 50% of the cohort below the age of 50 years had no additional comorbidity compared with around 25% of those aged 65 and older; conversely, fewer than 20% of those younger than 50 had two or more comorbidities in addition to HIV compared with over 30% of those aged 50–64 years and nearly 50% of the oldest age group[55]
.
Public Health England, an executive agency of the UK Department of Health, has projected that the prevalence of four common comorbidities (high cholesterol, hypertension, diabetes and ‘heart conditions’) in PLWH in the UK will increase significantly in the forthcoming decade, associated with the ageing population[56]
. Based on self-reported comorbidities in the 2014 ‘Positive Voices’ survey[57]
and population estimates[29]
, the number of PLWH with high cholesterol and/or hypertension is expected to more than double in the 15 years from 2013 to 2028, with prevalence increasing from 19% to 29% and from 13% to 21%, respectively; in the same period, prevalence of both diabetes and ‘heart conditions’ are projected to double to around 1 in 15 of PLWH[56]
.
Frailty is itself a distinct long-term condition and one of its key predictors in older PLWH is multimorbidity[22]
. The signs and symptoms of the five ‘frailty syndromes’ (i.e. falls, immobility, delirium, incontinence and susceptibility to medication side effects) are not unique to individuals with frailty[43]
, but the presence of one or more in a PLWH should prompt further investigation and/or closer monitoring. Different HIV cohorts, most of which also included those under 50 years of age, have identified frailty prevalence rates of up to 29%[22]
. This compares with general population estimates of 10% of those aged over 65 years, which increases dramatically to between a quarter and a half of those aged over 85 years[58]
. Therefore, as the average age of PLWH continues to increase, with the associated multimorbidity, it is likely that the prevalence of those living with frailty will also rise. Although routine population screening for frailty is not currently recommended, it may be beneficial for at-risk groups[43]
; following validation of a suitable tool, this could include PLWH and comorbidities.
Polypharmacy and other medication-related problems
In the Swiss Cohort study, fewer than 20% of those under the age of 50 years were taking any medicines apart from ART, compared with more than 60% of those aged ≥65 years[55]
. A large French prospective cohort study presented similar findings: in their ‘experienced ageing’ subgroup (7,025 PLWH aged over 50 years who had been diagnosed ≥13 years), 62.1% had ≥1 comorbidity and 71% were receiving ≥1 non-ART medicine[59]
. ART typically comprises three medicines, therefore, coupled with the high prevalence of multimorbidity, it is unsurprising that many older PLWH also experience polypharmacy (when this is defined simply as the number of medicines prescribed, usually at least five)[60]
.
However, it has been recognised that this is not the most meaningful way of defining the issue[61]
. While the risk of toxicities and potential drug–drug interactions inevitably increases with the number of medicines taken, it is important to distinguish between appropriate and inappropriate (problematic) polypharmacy[61]
. With appropriate polypharmacy, the combination of medicines has been optimised so that all are beneficial to the patient and the benefits of the combination are greater than the risks or adverse effects. Conversely, the harms of problematic polypharmacy outweigh the benefits; this is particularly likely to occur when there are multiple prescribers with no one responsible for regularly reviewing the whole combination of medicines for indication, dose, appropriateness, drug–drug interactions and side effects[62]
.
Drug–drug interactions
The aforementioned increased incidence of comorbidities and polypharmacy in older PLWH also amplifies the risk of drug–drug interactions (DDIs), including those with ART. Some antiretrovirals have the potential to cause a wide range of clinically significant DDIs, including with many medicines that are commonly prescribed in primary care, with some that can be purchased from a pharmacy or from general retail outlets, as well as with some herbal products, nutritional supplements and illicit drugs. DDIs form the basis of a high percentage of queries received by HIV specialist pharmacists[63],[64]
and, if not managed appropriately, can result in serious morbidity and therapeutic failure of either ART or concomitant medicines[65]
. It is recommended that antiretrovirals are added to the patient’s general practice medication record, although not all potential DDIs may be identified by prescribing software. Further interpretation of DDI alerts are often required and, in such cases, the HIV pharmacist should be contacted.
A detailed description of DDIs and their management is beyond the scope of this review, but it is vital that all pharmacists have some understanding of the underlying mechanisms so they can elicit relevant information and be vigilant for potential interactions. It is also essential to be aware of the most appropriate information sources for aiding identification of significant DDIs (see ‘Box 3: Useful reference sources’). The Summary of Product Characteristics and British National Formulary can be helpful, but specialist sites provide additional guidance[66]
. The University of Liverpool HIV Drug Interaction website, ‘Treatment Selectors’, provides regularly updated summaries to aid the management of specific conditions, including those more prevalent in older PLWH (e.g. hypertension, hyperlipidaemia and renal dysfunction). While this applies to the care and management of all PLWH, it is particularly pertinent to those who are older. Pharmacy staff may be less likely to identify ‘young-old’ PLWH as being at risk, since in the HIV-negative population the incidence of multimorbidity and polypharmacy (and associated heightened DDI risk) occurs on average at a much older age.
Box 3: Useful reference sources
Online drug interaction checkers:
- University of Liverpool HIV Drug Interactions;
- Toronto General Hospital Immunodeficiency Clinic;
- University of California San Francisco HIV InSite
NB These resources are for checking interactions between antiretroviral therapy (ART) and other medicines. They do not identify interactions between non-ART medicines.
HIV clinical guidelines:
HIV pharmacy advice and continuing professional development:
- HIV Pharmacy Association (HIVPA)
Some important DDIs that may be encountered by those outside the HIV specialty are outlined here, but this is not a comprehensive list.
The ART regimens most commonly involved in drug–drug interactions are those containing ritonavir or cobicistat, which are both used as pharmacokinetic enhancers to increase the concentration of protease inhibitors (PIs) or elvitegravir (an integrase inhibitor). PLWH who commenced ART in the 1990s (many of whom are now in the ‘ageing’ category) are more likely to be taking a PI-containing regimen than those who started more recently. This is partly due to the higher incidence of nucleoside resistance mutations (acquired following the use of sequential mono- and dual-ART in the pre-triple therapy era), coupled with the PIs’ higher genetic barrier to resistance compared with other antiretroviral classes. Additionally, for patients who have been taking effective ART since the mid-1990s (prior to the widespread availability of viral load and resistance testing), resistance may be presumed but unconfirmed, therefore, caution must be exercised when changing treatment.
Ritonavir and cobicistat are both primarily metabolised by, and potent inhibitors of, cytochrome P450 (CYP) 3A4; they are both also metabolised to a lesser degree by, and weaker inhibitors of, CYP2D6. In addition, they both affect various cellular membrane transporters (e.g. P-glycoprotein). However, ritonavir induces and inhibits a wider range of metabolic enzymes than cobicistat, so although their impact on most medicines is similar, there are important differences in their interactions with other drugs[67],[68]
. Caution is required, especially when switching from one to the other, or when trying to predict potential DDIs.
The challenges associated with DDIs between ART and concomitant medicines are amply illustrated by corticosteroids. Although these are widely used by all age groups, older PLWH carry a higher burden of relevant comorbidities than those who are younger[69]
, so it is likely that this DDI disproportionately affects them. The accessibility of corticosteroids, particularly ‘pharmacy only’ or ‘general sales list’ preparations, coupled with their wide range of indications and formulations, is problematic when trying to minimise DDIs.
Corticosteroids are metabolised by CYP3A4, so their levels are increased by PIs and pharmacokinetic enhancers, but decreased by most of the NNRTIs, necessitating appropriate dose adjustment. However, dexamethasone is also an inducer of CYP3A4 and thus reduces the blood levels of CYP3A4-metabolised antiretrovirals if chronically co-administered. Therefore, to avoid HIV treatment failure, dexamethasone (except for a single dose) should be avoided if possible in those who are taking non-nucleoside reverse transcriptase inhibitors (NNRTIs), PIs or elvitegravir/cobicistat. On the other hand, prednisolone and other corticosteroids have no effect on antiretroviral metabolism, so can be given with ART (with dose adjustment if necessary)[70]
.
In addition, the interaction between corticosteroids and ritonavir/cobicistat is uniquely significant, partly because it includes topical and intra-articular formulations, but also due to the severity of the potential consequences (i.e. iatrogenic Cushing’s syndrome and secondary adrenal insufficiency). Many cases have been attributed to intra-articular injection of triamcinolone or the use of fluticasone (nasal spray and inhaler formulations), plus one case of dexamethasone 0.1% eye drops with betamethasone 0.1% eye ointment. Where appropriate, beclomethasone should be used because this does not interact with any antiretrovirals. If this poses a challenge to the management of the condition, the HIV multidisciplinary team (MDT) or pharmacist should be contacted for advice; sometimes a change in ART is possible to avoid the DDI.
Finally, DDIs can occur as a result of preparations that increase gastric pH, or divalent cations that can cause chelation[71]
. These reduce the absorption of a number of antiretrovirals, including atazanavir, rilpivirine and the integrase inhibitors (e.g. dolutegravir, elvitegravir and raltegravir), with the potential to cause treatment failure and HIV resistance. These DDIs are particularly problematic because most of the offending agents (proton-pump inhibitors, H2 -receptor antagonists, antacids, vitamins, minerals and dietary supplements) can be obtained without prescription and are generally regarded as safe. Furthermore, the extent of the interaction and the specific management is different for each of the affected antiretrovirals. For example, all of the integrase inhibitors are affected by chelation but not by gastric acid suppression; however, the advice on choice and timing of co-administered minerals to avoid an adverse interaction differs for each of them[72]
. Conversely, absorption of atazanavir and rilpivirine requires an acidic pH, but chelation is not an issue. Therefore, proton pump inhibitors are contraindicated with both, but guidance differs on the choice, dose, frequency and timing of H2 -receptor antagonists and antacids with each of them[73]
.
Adherence
The importance of maintaining a consistently high level of adherence to ART has been an integral part of patient information and consultations in the UK for over 20 years, which has undoubtedly contributed to world-leading clinical outcomes[74]
, with 94% of PLWH who are on treatment achieving HIV RNA of <200 copies/ml[32]
. However, there is no room for complacency and a proactive approach is required now to ensure this record is sustained. Key factors associated with HIV and ageing that could negatively impact adherence and thus jeopardise future success have been identified above, including:
- Stigma, which may adversely affect mental health and increase social isolation;
- Problematic polypharmacy, due to multimorbidity;
- Increased incidence of neurocognitive degeneration/dementia;
- Multiple care providers, specifically in relation to prescribing, dispensing and monitoring of ART and other medicines (including non-prescription and herbal products).
In addition, as more antiretrovirals come off patent there will be opportunities to make significant financial savings by splitting fixed-dose combinations into multi-tablet regimens[75],[76]
. Therefore, it is vital that PLWH are active partners in commissioning and individual decision-making about such switches, to minimise the risk of precipitating a decrease in adherence, either intentional or unintentional[77]
.
Medicines optimisation
Specialist pharmacists have been at the heart of most HIV multidisciplinary teams since before the advent of ART, and many of the National Institute for Health and Care Excellence (NICE) Medicines Optimisation Guideline recommendations were already enshrined in the British HIV Association (BHIVA) Standards of Care[78],[79]
. However, new challenges have arisen in recent years, in addition to the demographic changes, partly due to the shift in prescribing of non-HIV medicines to primary care, and also because the majority of HIV outpatient dispensing has now been outsourced, either to homecare companies or to community pharmacies based in hospitals.
HIV specialist pharmacist roles in medicines optimisation include: assisting with treatment recommendations (e.g. as part of a multidisciplinary ‘virtual clinic’); patient consultations to initiate or switch ART (including prescribing); conducting medication reviews and responding to medicines-related queries from PLWH, as well as primary and secondary care colleagues.
Other pharmacists, including those in community and primary care settings, need to be alert to the potential for DDIs between ART and other medicines (prescribed and non-prescribed, including vitamin and mineral supplements, over-the-counter and herbal products). The main barrier in community pharmacy is likely to be lack of awareness of the individual’s HIV status.
Healthcare professionals involved in prescribing or screening/dispensing should have access to a complete, accurate drug history, and pharmacists are often well-placed to facilitate communication between different healthcare providers.
Models of care
The greatest challenge currently facing HIV management is establishing innovative, evidence-based, patient-centred models of health and social care that reflect the current and expected future needs of PLWH throughout their life course, and which can be delivered within the existing commissioning framework and budgetary constraints[80],[81]
. The primary issue driving both the requirement for change and the shape of future services is the ‘greying of HIV’[82]
, and the associated need to integrate the management of increasingly complex ‘general’ health and social care issues with more stable, less intensive specialist input.
However, notwithstanding the tremendous progress in HIV diagnosis, monitoring and treatment, stigma (including self-stigma and stigma in healthcare settings) remains a major concern for all demographic groups, and has been shown to contribute to delays in testing, suboptimal adherence, disengagement with care and psychological distress. The National AIDS Trust highlighted the paucity of evidence-based, multi-faceted interventions to reduce HIV stigma in the UK, but emphasised the importance of implementing and evaluating a range of interventions, including in healthcare settings[83]
. The King’s Fund and BHIVA both echoed this recommendation, stressing the need for a national education programme that involves PLWH[80],[81]
. For example, PLWH need to be sure that confidentiality will be respected by everyone involved in their care (including administration and support staff), and that they will not experience prejudice and discrimination. This applies to community pharmacies, nursing and residential care homes, as well as general practice, dentists and other primary and secondary care providers. Any proposed model of care must be cognisant of, and address, stigma, if it is to be patient-centred and sustainable.
Who currently manages the care of PLWH?
The HIV specialty has always had the MDT at the heart of patient care, and professional roles have evolved and adapted as models of care and the needs of PLWH have changed. Some centres have comorbidity clinics, run jointly by consultants from HIV and other specialties (e.g. renal). Clinics for PLWH over the age of 50 years, or those who are clinically complex, have also been established.
In the UK, PLWH who are stable on ART are typically followed up every 6–12 months by their HIV outpatient clinician, who is responsible for prescribing and managing their ART and any other directly HIV-related medicines (e.g. co-trimoxazole for Pneumocystis jiroveci prophylaxis). Blood tests (i.e. viral load and routine toxicity monitoring, e.g. full blood count, hepatic and renal function) are performed every six months, with results fed back to the patient by email or telephone if they are only seeing their HIV specialist once a year. CD4 count blood tests are no longer required if the patient remains on ART, having had CD4 >350 cells/mm3 and fully suppressed viral load on at least two consecutive occasions, at least one year apart[24]
. Other specialist treatments (e.g. for hepatitis C or for cancers) are managed by the appropriate secondary or tertiary care teams.
Gender-specific issues that are more common in older people (i.e. primarily low testosterone and the menopause) are managed in primary care, in accordance with usual best practice and/or national guidelines[84]
. Low testosterone appears to be more common in men with HIV, particularly those not on effective ART[85]
. It is unclear whether HIV affects the timing and symptoms of the menopause, but it is recommended that women with HIV be questioned about their menstrual cycle and menopausal symptoms annually from the age of 45 years[86]
. GPs are usually responsible for the ongoing management of all other medicines, including those recommended or initiated by most non-HIV secondary care specialties, as well as screening tests (e.g. cervical smears) and routine immunisations (e.g. influenza and pneumococcal vaccines).
The resulting lack of a unified dispensing record increases the probability of potentially inappropriate or interacting medicines remaining undetected. Older PLWH, with their higher likelihood of having multimorbidity and multiple prescribers involved, are therefore particularly at risk of problematic polypharmacy under the present arrangements. To mitigate this danger, GPs are advised to add antiretrovirals to the patient’s electronic record, ensuring that they cannot be prescribed by the practice, so that most DDIs with GP-prescribed medicines will be highlighted[81]
.
The BHIVA Standards of Care and Monitoring Guidelines both also address this safety concern, mandating that a drug history should be taken at each clinic visit, with a full medication review conducted annually by a specialist HIV pharmacist[24],[79]
but compliance with the latter is by no means universal, due to financial and capacity constraints (and hindered by the fragmentation of HIV and sexual health commissioning since 2012). Unfortunately, care co-ordination and management of non-HIV-related conditions fall between the remits of NHS England and clinical commissioning groups[87]
; therefore, for example, specialist pharmacists based in secondary care cannot usually be reimbursed for conducting medicines use reviews on PLWH.
Future models of care
Two recent reports from BHIVA and the King’s Fund have showcased examples of innovative models of HIV care, and have articulated the barriers and facilitators to developing patient-centred collaborative care pathways that will build on the strengths of existing systems, as well as addressing their limitations[80],[81]
.
One crucial challenge is the readiness and confidence of primary care teams. This was highlighted by a recent survey of 250 GPs in England, which found that 41% were not confident managing the primary care needs of PLWH, ranging from 26% in areas of very high HIV prevalence to 48% in low prevalence areas[88]
Of those surveyed, 52% felt that they were less knowledgeable about HIV considerations and less supported as a GP to manage the primary care needs of people with HIV, compared with other long-term chronic conditions. Of those surveyed, 52% felt that they were less supported as a GP to manage the primary care needs of people with HIV compared with other long-term chronic conditions and that they were less knowledgeable about HIV considerations compared with other long-term chronic conditions. Of particular relevance to pharmacy professionals is that only 16% felt confident about their knowledge of HIV drug–drug interactions, ranging from 12% of respondents in areas with a low prevalence of HIV to 30% of those in very high prevalence areas.
The HIV-Ed course (developed in Brighton and subsequently adopted in other areas), along with the willingness of specialist pharmacists to answer queries by telephone or email, are part of a multi-pronged approach to address these issues.
Different models of care are likely to be required to meet local needs, and further evaluation and research will be required to identify the most appropriate ones, focusing on clinical outcomes, cost-effectiveness and patient satisfaction[81]
. Mechanisms for addressing health and social care needs locally/regionally with local authorities and the NHS were detailed in the King’s Fund Report[80]
. These include joint strategic needs assessments, joint health and wellbeing strategies and Sustainability and Transformation Plans[80]
.
Although most pharmacists are unlikely to be involved in these processes, and detailed discussion of them is beyond the scope of this review, there will still be opportunities to help drive or participate in initiatives as part of their local HIV centre or network. Pharmacists working in general practice, community and hospital sectors should be involved in establishing local pathways to facilitate clear, timely communication about medicines-related issues that meet the needs of PLWH and local health services.
Conclusion
The aetiology of ageing and HANA conditions in PLWH is complex and involves several elements, including accelerated and accentuated ageing, higher rates of traditional lifestyle and behavioural risk factors, and (to a lesser extent) antiretroviral treatment. Multimorbidity, polypharmacy and medication-related problems, especially DDIs, are more common in older PLWH than in both those who are younger or in their contemporaries without HIV. The challenges resulting from a combination of these factors and the previously described demographic changes, has been a major influence on the current drive to explore new models of care for PLWH. The development of cost-effective, integrated, patient-centred, collaborative care pathways for HIV health and social care must be a priority if clinical outcomes and patient satisfaction are to be maintained or improved as the population ages[80]
.
Acknowledgment:
The author would like to acknowledge the significant contribution of the late Professor Martin Fisher to the field of HIV and ageing, and to her own understanding of it.
Author disclosures and conflicts of interests:
The author is an HIVPA Expert Panel member, NHS England HIV Clinical Reference Group member (and HIV Drugs Sub-group co-chair). In the past five years the author has received honoraria, unrestricted educational grants or research funding from the following pharmaceutical companies: Gilead, Janssen, MSD and ViiV. No writing assistance was used in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Centers for Disease Control and Prevention(CDC). Pneumocystis pneumonia-Los Angeles. MMWR Morb Mortal Wkly Rep. 1981; 30(21):250-252. Available at: https://stacks.cdc.gov/view/cdc/1261/ (accessed February 2018)
[2] Department of Social and General Statistics. HIV and AIDS statistics. SN/SG/2210. London: House of Commons Library; 2012. [Online] Available at: http://researchbriefings.parliament.uk/ResearchBriefing/Summary/SN02210 (accessed February 2018)
[3] Siminoff LA, Erlen JA & Lidz CW. Stigma, AIDS and quality of nursing care: state of the science. J Adv Nurs. 1991;16(3):262–269. doi: 10.1111/j.1365-2648.1991.tb01648.x
[4] Barré-Sinoussi F, Chermann JC, Rey F et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983;220(4599):868–871. doi: 10.1126/science.6189183
[5] Kalyanaraman VS, Cabradilla CD, Getchell JP et al. Antibodies to the core protein of lymphadenopathy-associated virus (LAV) in patients with AIDS. Science 1984;225(4659):321–323. doi: 10.1126/science.6330889
[6] Palella FJ, Delaney KM, Moorman AC et al.; the HIV Outpatient Study Investigators. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998;338:853–860. doi: 10.1056/NEJM199803263381301
[7] Revets H, Marissens D, de Wit S et al. Comparative evaluation of NASBA HIV-1 RNA QT, AMPLICOR-HIV monitor, and QUANTIPLEX HIV RNA assay, three methods for quantification of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1996;34(5):1058–1064. PMID: 8727875
[8] Clevenbergh P, Durant J, Halfon P et al. Persisting long-term benefit of genotype-guided treatment for HIV-infected patients failing HAART. The Viradapt Study: week 48 follow-up. Antivir Ther 2000;5(1):65–70. PMID: 10846595
[9] John Henkel. Attacking AIDS with a ‘cocktail’ therapy: drug combo sends deaths plummeting. U.S. Food and Drug Administration. Available at: http://www.thebody.com/content/art13838.html (accessed February 2018)
[10] Paterson DL, Swindells S, Mohr J et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00025
[11] Pennings PS. HIV drug resistance: problems and perspectives. Infect Dis Rep 2013;6(5,Suppl 1):e5. doi: 10.4081/idr.2013.s1.e5
[12] Freedberg KA, Losina E, Weinstein MC et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med 2001;344:824–831. doi: 10.1056/NEJM200103153441108
[13] Bansi L, Sabin C, Delpech V et al.; for the UK Collaborative HIV Cohort (CHIC) Study and the Health Protection Agency. Trends over calendar time in antiretroviral treatment success and failure in HIV clinic populations. HIV Medicine 2010;11:432–438. doi: 10.1111/j.1468-1293.2009.00809.x
[14] Public Health England (PHE). Chau C, Kirwan P, Brown A, Gill N and Delpech V. HIV in the United Kingdom: decline in new HIV diagnoses in gay and bisexual men in London, 2017 report. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/557339/HIV_diagnoses__late_diagnoses_and_numbers_accessing_treatment_and_care_NB300916V2.pdf (accessed February 2018)
[15] Public Health England (PHE). Recent trends in life expectancy at older ages: Update to 2014. Available at: https://www.gov.uk/government/publications/life-expectancy-recent-trends-in-older-ages (accessed February 2018)
[16] Sabin C. Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Medicine 2013;11:251–257. doi: 10.1186/1741-7015-11-251
[17] British HIV Association (BHIVA). BHIVA guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015 (2016 interim update). Available at: http://www.bhiva.org/HIV-1-treatment-guidelines.aspx (accessed February 2018)
[18] World Health Organization (WHO). Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Available at: http://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/ (accessed February 2018)
[19] Serrano-Villar S, Gutiérrez F, Miralles C et al. Human immunodeficiency virus as a chronic disease: evaluation and management of nonacquired immune deficiency syndrome-defining conditions. Open Forum Infect Dis 2016;3(2):97–111. doi: 10.1093/ofid/ofw097
[20] Sangarlangkarn A & Appelbaum JS. Caring for older adults with the human immunodeficiency virus. J Am Geriatr Soc 2016;64(11):2322–2329. doi: 10.1111/jgs.14584
[21] World Health Organization (WHO). Proposed working definition of an older person in Africa for the MDS Project. Available at: http://www.who.int/healthinfo/survey/ageingdefnolder/en/ (accessed February 2018)
[22] Levett TJ, Cresswell FV, Malik MA et al. Systematic review of prevalence and predictors of frailty in individuals with human immunodeficiency virus. J Am Geriatr Soc 2016;64:1006–1014. doi: 10.1111/jgs.14101
[23] American Academy of HIV Medicine. The HIV and aging consensus project: recommended treatment strategies for clinicians managing older patients with HIV. Available at: https://aahivm.org/wp-content/uploads/2017/02/Aging-report-working-document-FINAL-12.1.pdf (accessed February 2018)
[24] British HIV Association (BHIVA). BHIVA guidelines for the routine investigation and monitoring of adult HIV-1-positive individuals (2016). Available at: http://www.bhiva.org/monitoring-guidelines.aspx (accessed February 2018)
[25] Mosby’s Medical Dictionary, 9th edition. Available at: http://medical-dictionary.thefreedictionary.com (accessed February 2018)
[26] Cahill S & Valadéz R. Growing older with HIV/AIDS: new public health challenges. Am J Public Health 2013;103(3):e7–e15. doi: 10.2105/AJPH.2012.301161
[27] Beer G, James M & Summers S. Growing older positively: the challenge of ageing with HIV. Available at: http://www.2020health.org/2020health/Publications/Publications-2014/HIV.html (accessed February 2018)
[28] Public Health England (PHE). National HIV surveillance data tables. Tables No. 1: 2016 (correction March 2017). Available at: https://www.gov.uk/government/statistics/hiv-annual-data-tables (accessed February 2018)
[29] Yin Z, Kall M, Skingsley A et al. ‘Over half of people in HIV care in the UK by 2028 will be aged 50 years or above’. Presentation at the 15th European AIDS Conference, 21–24 October 2015, Barcelona, Spain. Available at: http://www.ucl.ac.uk/voices/pdfs/HIV_care (accessed February 2018)
[30] The Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017;4:e349–356. doi: 10.1016/S2352-3018(17)30066-8
[31] Kirwan P, Brown A, Connor N & Delpech V. Use of HIV public health monitoring data to redefine thresholds for expanded HIV testing in England. Research and Applied Epidemiology Scientific Conference 2017, Warwick University. Available at: https://www.researchgate.net/publication/316084962_Use_of_HIV_public_health_monitoring_data_to_redefine_thresholds_for_expanded_HIV_testing_in_England (accessed February 2018)
[32] Public Health England. Kirwan PD, Chau C, Brown AE at al. HIV in the UK – 2016 report. December 2016. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/602942/HIV_in_the_UK_report.pdf (accessed February 2018)
[33] Davis DHJ, Smith R, Brown A et al. Early diagnosis and treatment of HIV infection: magnitude of benefit on short-term mortality is greatest in older adults. Age Ageing 2013;42:520–526. doi: 10.1093/ageing/aft052
[34] Harris J & Khatri R. Late diagnosis of HIV in the United Kingdom: An evidence review. Public Health Institute, Liverpool John Moores University. Available at: http://www.cph.org.uk/publication/late-diagnosis-of-hiv-in-the-united-kingdom-an-evidence-review/ (accessed February 2018)
[35] Rosenfeld D, Bartlam B & Smith RD. Out of the closet and into the trenches: gay male baby boomers, aging, and HIV/AIDS. Gerontologist 2012;52(2):255–264. doi: 10.1093/geront/gnr138
[36] thewellproject. Available at: http://www.thewellproject.org/hiv-information/long-term-survivors-hiv (accessed February 2018)
[37] Owen G & Catalan J. ‘We never expected this to happen’: narratives of ageing with HIV among gay men living in London, UK. Cult Health Sex 2012;14(1):59–72. doi: 10.1080/13691058.2011.621449
[38] McGowan JA, Sherr L, Rodger AJ et al.; the Antiretrovirals, Sexual Transmission Risk and Attitudes (ASTRA) Study Group. Age, time living with diagnosed HIV infection, and self-rated health. HIV Medicine 2017;18:89–103. doi: 10.1111/hiv.12398
[39] High KP, Brennan-Ing M, Clifford DB et al.; the OAR Working Group on HIV and Aging. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012;60:S1–18. doi: 10.1097/QAI.0b013e31825a3668
[40] Brothers TD & Rockwood K. Biologic aging, frailty, and age-related disease in chronic HIV infection. Curr Opin HIV AIDS 2014;9:412–418. doi: 10.1097/COH.0000000000000070
[41] De Biasi S, Pinti M, Nasi M et al. HIV-1 Infection and the aging of the immune system: facts, similarities and perspectives. J Exp Clin Med 2011;3(4):143–150. doi: 10.1016/j.jecm.2011.06.001
[42] Leng SX & Margolick JB. Understanding frailty, aging, and inflammation in HIV infection. Curr HIV/AIDS Rep 2015;12(1):25–32. doi: 10.1007/s11904-014-0247-3
[43] British Geriatrics Society. Fit for frailty: consensus best practice guidance for the care of older people living with frailty in community and outpatient settings. Available at: http://www.bgs.org.uk/index.php/resources-6/bgscampaigns/fit-for-frailty (accessed February 2018)
[44] Pathai S, Bajillan H, Landay AL & High KP. Is HIV a model of accelerated or accentuated aging? J Gerontology 2014;69(7):833–842. doi: 10.1093/Gerona/glt168
[45] Fisher M & Cooper V. HIV and ageing: premature ageing or premature conclusions. Curr Opin Infect Dis 2012;25:1–3. doi: 10.1097/QCO.0b013e32834f14fa
[46] Zapata HJ & Shaw AC. Aging of the human innate immune system in HIV infection. Curr Opin Immunol 2014;29:127–136. doi: 10.1016/j.coi.2014.06.007
[47] Gross AM, Jaeger PA, Kreisberg JF et al. Methylome-wide analysis of chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell 2016;62(2):157–168. doi: 10.1016/j.molcel.2016.03.019
[48] Horvath S & Levine AJ. HIV-1 Infection accelerates age according to the epigenetic clock. J Infect Dis 2015;212:1563–1573. doi: 10.1093/infdis/jiv277
[49] Petrosillo N & Cicalini S. Smoking and HIV: time for a change? BMC Med 2013;11:16. doi: 10.1186/1741-7015-11-16
[50] Hunt PW. HIV and ageing: emerging research issues. Curr Opin HIV AIDS 2014;9(4):302–308. doi: 10.1097/COH.0000000000000072
[51] Smith RL, de Boer R, Brul S et al. Premature and accelerated aging: HIV or HAART? Front Genet 2013;3:328–337. doi: 10.3389/fgene.2012.00328
[52] El-Sadr WM, Lundgren J, Neaton JD et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360
[53] Centre of Excellence for Health, Immunity and Infections. Data Collection on Adverse Events of Anti-HIV Drugs (the D:A:D Study). Available at: https://www.chip.dk/Studies/DAD/Presentations/2017 (accessed February 2018)
[54] Guaraldi G, Orlando G, Zona S et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011;53(11):1120–1126. doi: 10.1093/cid/cir627
[55] Hasse B, Ledergerber B, Furrer H et al.; the Swiss HIV Cohort Study. Morbidity and aging in HIV-infected persons: the Swiss HIV Cohort Study. Clin Infect Dis 2011;35(11):1130–1139. doi: 10.1093/cid/cir626
[56] Kall M, Yin Z, Skingsley A et al.; on behalf of the Positive Voices Study Group. Chronic disease in the HIV population in care in the UK: projections until 2028. 17th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV. Barcelona, Spain; October 20-22 2015. Available at: http://www.ucl.ac.uk/voices/pdfs/Chronic_disease (accessed February 2018)
[57] Kall M, Shahmanesh M, Nardone A et al.; on behalf of the Positive Voices Study Group. Self-reported prevalence of co-morbidities and use of non-HIV related medications among people living with HIV in England and Wales: results from the Positive Voices survey. 15th European AIDS Conference. Barcelona, Spain; October 21–24 2015. Available at: http://www.ucl.ac.uk/voices/pdfs/Co_morbidities (accessed February 2018)
[58] Clegg A, Young J, Iliffe S et al. Frailty in elderly people. Lancet 2013;381(868):752–762. doi: 10.1016/s0140-6736(12)62167-9
[59] Cuzin L, Katlama C, Cotte L et al. Ageing with HIV: do comorbidities and polymedication drive treatment optimization? HIV Med 2017;18(6):395–401. doi: 10.1111/hiv.12441
[60] The King’s Fund. Duerden M, Avery T & Payne R. Polypharmacy and medicines optimisation: making it safe and sound. Available at: https://www.kingsfund.org.uk/publications/polypharmacy-and-medicines-optimisation (accessed February 2018)
[61] Patterson SM, Cadogan CA, Kerse N et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2014;10:CD008165. doi: 10.1002/14651858.CD008165.pub3
[62] PrescQIPP. Shaw V (ed.) Ensuring appropriate polypharmacy. Bulletin 136. 2016. Available at: https://www.prescqipp.info/polypharmacy-ensuring-appropriate-polypharmacy/send/275-polypharmacy-ensuring-appropriate-polypharmacy/2553-bulletin-136-polypharmacy-and-deprescribing (accessed February 2018)
[63] Marshall N, Hedley L, Lenko A et al. Indispensable? The questions healthcare professionals ask specialist HIV pharmacy services (Poster P19). Presented at the Third Joint Conference of BHIVA with BASHH: British HIV Association. HIV Med 2014;15(Suppl. 3):17–159. Available at: http://www.bhiva.org/documents/Conferences/2014Liverpool/AbstractBook2014.pdf (accessed February 2018)
[64] Marshall N, Shahbakhti H, Lenko A et al. Questions about medicines: how specialist HIV pharmacy services are utilised (Poster P11). Presented at the Third Joint Conference of BHIVA with BASHH: British HIV Association. HIV Med 2014; 15(Suppl. 3):17–159. Available at: http://www.bhiva.org/documents/Conferences/2014Liverpool/AbstractBook2014.pdf (acessed February 2048)
[65] Fasinu PS, Gurley BJ & Walker LA. Clinically relevant pharmacokinetic herb–drug interactions in antiretroviral therapy. Curr Drug Metab 2016;17:52–64. doi: 10.2174/1389200216666151103115053
[66] Marshall N, Sonecha S, Okoli C et al. Do common medicines information resources identify drug interactions between the most frequently prescribed medicines in primary care in the UK and antiretrovirals? (Poster P316) Presented at the Third Joint Conference of BHIVA with BASHH: British HIV Association. HIV Med 2014;15(Suppl. 3):17–159. Available at: http://www.bhiva.org/documents/Conferences/2014Liverpool/AbstractBook2014.pdf (accessed February 2018)
[67] Electronic Medicines Compendium. Tybost 150mg film coated tablet: Summary of Product Characteristics [Online] Available at: https://www.medicines.org.uk/emc/medicine/28298 (accessed February 2018)
[68] Electronic Medicines Compendium. Norvir 100mg film-coated tablets: Summary of Product Characteristics [Online] Available at: http://www.medicines.org.uk/EMC/medicine/22952 (accessed February 2018)
[69] Elliot ER, Theodoraki A, Jain LR et al. Iatrogenic Cushing’s syndrome due to drug interaction between glucocorticoids and the ritonavir or cobicistat containing HIV therapies. Clin Med (Lond) 2016;16(5):412–418. doi: 10.7861/clinmedicine.16-5-412
[70] University of Liverpool HIV drug interactions checker. Treatment selector: corticosteroids. Available at: http://hiv-druginteractions.org/treatment_selectors (accessed February 2018)
[71] University of California San Francisco. Center for HIV Information. Interactions between gastrointestinal secretion and motility agents and antiretrovirals. Available at: http://hivinsite.ucsf.edu/insite?page=ar-00-02&post=10¶m=2 (accessed February 2018)
[72] US Department of Veterans Affairs. Coffey S. Drug Interactions: integrase inhibitors and cations. Available at: http://www.hiv.va.gov/provider/hivmeds-quarterly/2014/integrase-inhibitors-cations.asp (accessed February 2018)
[73] University of California San Francisco. Center for HIV Information. Jacobson MA, Luetkemeyer A & Wlodarczyk D. Potential Interactions between acid-blocking medications and ARVs in managing an HIV patient with odynophagia or dysphagia. Available at: http://hivinsite.ucsf.edu/InSite?page=md-ward86-odynophagia (accessed February 2018)
[74] Levi J, Raymond A, Pozniak A et al. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob Health 2016;1(2),e000010. doi: 10.1136/bmjgh-2015-000010
[75] British HIV Association (BHIVA). Hardweir V, Simpkin E, Leake Date H et al. The next generiction update. 23rd Annual Conference of BHIVA, Liverpool. 5th April 2017. Abstract O6 HIV Med 2017;18(Suppl. 1). Available at: http://bhiva.org/170405VenitaHardweir.aspx (accessed February 2018)
[76] NHS England. Heart of England NHS Foundation Trust. Midlands and east region: antiretroviral therapy (ART) prescribing implementation guidance for adult and adolescent patients starting and switching treatment 2017. Available at: http://www.hivbirmingham.nhs.uk/professional/regional-guidelines/ (accessed February 2018)
[77] Annandale D & Leake Date HA. Failure modes effects analysis (FMEA): could it be used to reduce the risks associated with antiretroviral (ARV) formulation changes (FCs)? 2nd Joint Conference of BHIVA with BASHH, Manchester, April 2010. Abstract P19 in: HIV Medicine 11(Suppl. 1):25. doi: 10.1111/j.1468-1293.2010.00841_2.x
[78] National Institute for Health and Care Excellence (NICE). Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes. NICE guideline [NG5]. Available at: www.nice.org.uk/guidance/ng5 (accessed February 2018)
[79] British HIV Association (BHIVA). Standards of Care for People Living with HIV. London: Mediscript; 2012. Available at: http://www.bhiva.org/standards-of-care-2013.aspx (accessed February 2018)
[80] The King’s Fund. The future of HIV services in England: shaping the response to changing needs. Available at: https://www.kingsfund.org.uk/publications/future-hiv-services-england (accessed February 2018)
[81] MacLellan J, Shahmanesh M, Singh S et al. Shared care: how can we do it? Findings from the BHIVA Primary Care Project. London: Mediscript; 2017. Available at: http://www.bhiva.org/shared-care.aspx (accessed February 2018)
[82] Harris CM, McKenzie R, Nayak S et al. Graying of the HIV epidemic: a challenge for inpatient medicine providers. J Community Hosp Intern Med Perspect 2015;5(6):29428. doi: 10.3402/jchimp.v5.29428
[83] National AIDS Trust. Tackling HIV stigma: what works? Using the global evidence base to reduce the impact of HIV stigma. Available at: http://www.nat.org.uk/publications (accessed February 2018)
[84] National Institute for Health and Care Excellence (NICE). NICE guideline [NG23]. Menopause: diagnosis and management. Available at: https://www.nice.org.uk/guidance/ng23 (accessed February 2018)
[85] Ashby J, Goldmeier D & Sadeghi-Nejad H. Hypogonadism in human immunodeficiency virus-positive men. Korean J Urol 2014;55(1):9–16. doi: 10.4111/kju.2014.55.1.9
[86] Waters L, Lord E, Mackie N et al. BHIVA/BASHH/FSRH guidelines for the sexual & reproductive health of people living with HIV. Available at: http://www.bhiva.org/SRH-guidelines-consultation.aspx (accessed February 2018)
[87] Legislation.gov.uk. Health and Social Care Act 2012. Chapter 7. Available at: http://www.legislation.gov.uk/ukpga/2012/7/contents (accessed February 2018)
[88] British HIV Association (BHIVA). Forni J. HIV Stigma and Discrimination in Primary Care [Presentation] 22nd Annual Conference of BHIVA, Manchester. 20th April 2016. Abstract O6. HIV Med 2016;17(Suppl. 1). Available at: http://bhiva.org/AnnualConference2016.aspx (accessed February 2018)