Key points
- Pharmacist involvement in post-take ward rounds is part of an established service at Imperial College Healthcare NHS Trust;
- The purpose of this service evaluation was to determine the type and number of contributions a pharmacist makes on an acute medicine ward round;
- Overall, 65% (n=155) of 239 errors resulting in a classifiable contribution by the pharmacist were deemed to be ‘significant’ to ‘potentially lethal’. In addition, 70% (n=168) of a total of 239 classifiable contributions were thought to be ‘significant’ to ‘very significant’.
Introduction
A post-admission ward round, also referred to as a post-take ward round (PTWR), is a key component of daily hospital activity and provides an opportunity for the multidisciplinary team (MDT) to meet and review a patient’s condition. This often includes confirming diagnoses, refining management plans and initiating attempts to optimise the patient’s pharmacotherapy. The acute admissions unit is considered the gateway to the hospital, and the benefits of ward rounds, particularly the interventions and recommendations made on the PTWR, are likely to result in better patient outcomes on downstream wards[1]
.
Imperial College Healthcare NHS Trust (ICHNT) provides acute and specialist healthcare in north west London for around 1.5 million people every year, with 299,000 emergency attendees seen in 2017/2018[2]
. PTWRs within ICHNT assess patients within 24 hours of admission, and the MDT — including the consultant, specialist registrar, core trainee doctors, pharmacist and nurse in charge — decides whether to initiate treatment, transfer the patient to an appropriate ward or discharge them. Two PTWRs take place each day, at 08:00 and 15:00.
The current pharmacy service on the acute medical ward at ICHNT includes one pharmacist at St Mary’s Hospital and two pharmacists on each PTWR at Charing Cross Hospital (Agenda for Change band 6–8a). The accident and emergency services with acute medicine provision were included in this evaluation because they are based on these two sites.
On average, three hours (40%) of the pharmacists’ working day is spent on the PTWR; therefore, this service evaluation aimed to determine the contributions pharmacists made and the value a panel of senior practitioners associated with each type of contribution. The panel consisted of a senior pharmacist and two acute medicine consultants.
On PTWRs, pharmacists contribute to clinical decision-making through detection and prevention of medication errors, undertake medicines optimisation and reconciliation, and review pre-admission medication[2]
. Pharmacist involvement and input at the time of admission can reduce medication errors before medicines are administered, improve the timeliness of supply and ensure timely interventions[3]
.
Studies carried out on inpatient wards have revealed that areas with an older patient population are more likely to be at risk of medication errors, owing to the number of prescriptions that are issued as a result of multiple comorbidities[4],[5],[6]
. Winter pressures place great strain on the healthcare system and NHS England’s report ‘Pharmacy and medicines optimisation: a toolkit for winter 2018/19’ outlines the role of pharmacists, including in medication reviews and early identification of medication-related issues to avoid re-admissions[7]
.
A comparison of pharmacist interventions made on ward rounds, versus on a ward visit, by Miller et al., has shown that 1.73 interventions, compared with 0.89 interventions per patient, were made, respectively; however, these interventions were similar in nature and importance. The time to intervene, and for the intervention to be accepted by the clinician, was shorter on the ward round owing to the presence of the consultant, who is the primary decision-maker[8]
. In addition, pharmacist presence on an internal medicine ward round has been demonstrated to help contribute to rationalisation of drug therapy and lead to increased medicines safety[9]
.
A Royal Pharmaceutical Society report ‘Keeping patients safe when they transfer between care providers — getting the medicines right’ has outlined the steps required to improve the care between community and hospital discharge, as an estimated 30–70% of patients experience an unintentional change or error to their medicines on transfer. In many cases, these errors are avoidable and can lead to re-admissions to hospital[10]
. A pharmacist’s ability to carry out timely reconciliation on admission and discharge may allow for this to be improved[11]
.
Long-term national plans for clinical pharmacists have been outlined in the Carter report in 2016, and later analysed to show hospitals’ uptake of the recommendation[12]
. This service evaluation highlights the key recommendations of this report in practice, such as electronic prescribing, upskilling pharmacists and more time spent undertaking clinical activities.
The contributions a pharmacist can make at ward level (e.g. change of doses; advising on therapeutic drug monitoring) have been well defined[13],[14]
. An intervention defined in a similar setting by Overhage and Lukes includes “any form of communication between a pharmacist and a physician carried out with the intention of influencing prescribing”[15]
. However, this definition omits any contribution that does not directly affect the prescribing, such as providing advice to nurses on administration or patient interaction.
In this service evaluation, the definition of contribution has been extended to include interventions and all other processes that could, directly or indirectly, benefit a patient or the service.
Aims and objectives
This service evaluation was designed to:
- Identify prescribing errors and the type of contributions made by pharmacists on PTWRs;
- Determine the severity of medication errors based on the potential harm avoided, as rated by a panel of three senior members of the medical and pharmacy team;
- Determine the number of contributions a pharmacist makes on PTWRs;
- Establish the perceived value of the pharmacists’ contributions on PTWRs through a panel rating;
- Make recommendations on how the pharmacy service can be developed in the future.
The evaluation also provided an opportunity to increase awareness of the pharmacist’s role within the wider MDT and improve knowledge of common prescribing errors.
Methodology
Setting
Nine pharmacists — ranging in experience from Agenda for Change band 6 to band 8a — were involved in this work; each day during the two-week period, three pharmacists took part (based on rotas). Between 08:00 to 11:00 from Monday to Friday for ten days between 20 November 2017 and 1 December 2017, nine pharmacists collected prospective data on the PTWR on a rotational basis from multiple wards at St Mary’s Hospital and Charing Cross Hospital.
Approval
Trust and departmental approval were received before this service evaluation started.
Inclusion criteria and exclusion criteria
All adult patients aged over 18 years on the PTWR list in the emergency department, on wards and outliers were included, and all contributions made after PTWRs and on weekends, owing to different working hours, were excluded.
Data collection
A contribution was defined as “any form of communication between a pharmacist and a physician carried out with the intention of influencing prescribing and all other processes that could indirectly or directly benefit a patient or the service”[8]
.
A data collection tool was constructed, and a pilot carried out on the PTWR on 16–17 October 2017. Before starting the data collection, pharmacists were provided with guidance on collecting data and key definitions.
The following information was noted on the tool in real time:
- Number of patients on the PTWR (total and seen);
- Bed number;
- The number of active prescriptions before and after reviewing the electronic prescriptions on Cerner PowerChart, a prescribing system (including regular, as required and stat doses);
- Where applicable, the contribution and the associated drug name, strength and dosage;
- The outcome of the contribution;
- Whether the drug involved in the contribution was prescribed by the pharmacist.
Feedback following the pilot allowed for amendments to be made to permit the details of the contributions to be elaborated upon with minimal disruption to the PTWR.
On average, 40 patients were included on the PTWR list per day across both sites; therefore, the decision was made to carry out data collection over ten consecutive weekdays during a two-week period.
Contributions
Each contribution recorded on the data collection tool was uploaded manually to an Excel spreadsheet. Similar contributions were grouped together, after which the data were further classified into ‘type of contribution’.
Nine pharmacists ranging from band 6 to band 8a collected data. Pharmacists collected data from 08:00 to 11:00 on the Charing Cross Hospital site for two ward rounds, and one pharmacist worked on the St Mary’s Hospital site from 08:00 to midday.
The individual contributions (after removal of duplicate data entries, such as “venous thromboembolism risk assessment form prompted for completion”, but accounting for them quantitatively) were presented to an independent panel consisting of a senior pharmacist and two acute medicine consultants.
Contributions were evaluated by the panel using an adapted scale to rate the severity of medication errors and attribute a ‘value’ to the intervention made by the pharmacist (see Table 1 and Table 2)[9],[15],[17]
. Examples of interventions were included to ensure consistent ratings among the panel members[7]
. The three sets of ratings were then evaluated by the lead author (NP), to produce a single score by taking the average rating. This evaluation assumes that where contributions were recorded, errors were made and that the contribution from the pharmacist prevented the error from persisting, therefore reducing the overall probability of harm[16]
.
| Table 1. Scale used to determine the severity of medication errors | |
|---|---|
| Scale | Severity of error/event |
| A. Potentially lethal | Potentially life-saving medicine prescribed at a dose too low for the disease being treated |
| B. Serious | High dosage (4–10 times normal) with narrow therapeutic window |
| C. Significant | High dosage (1.5–4 times normal) with narrow therapeutic index |
| D. Minor | Incomplete information in medication order |
| E. No error | Information or clarification requested by the multidisciplinary team or cost-saving measures |
| Z. No intervention | Does not directly affect prescribing as per the definition from this service evaluation |
| Sources: Pharmacy World & Science [9] , American Journal of Health-System Pharmacy [15] , Drug Safety [17] | |
| Table 2. Scale used to determine the perceived value of the pharmacist intervention | |
|---|---|
| Scale | Value of the pharmacist intervention (with examples) |
| 1. Extremely significant | Recommendation for extremely serious or potential life-and-death situation |
| 2. Very significant | Recommendation for a potential or existing dysfunction in a major organ |
| 3. Significant | Recommendation brings about an acceptable level of care (standard practice) |
| 4. Somewhat significant | Patient benefiting from the recommendation could be neutral depending on interpretation |
| 5. No significance | Information only or recommendation is not specific to the patient |
| 6. Inappropriate | Recommendation inappropriate or lead to poor outcomes |
| 7. No intervention | Not applicable |
| Sources: Pharmacy World & Science [9] , American Journal of Health-System Pharmacy [15] , Drug Safety [17] | |
Results
A total of 312 patients were included on the PTWR lists across both sites over the two-week study period; 247 (79%) were seen by a pharmacist. A total of 65 patients (21%) were not seen by a pharmacist on the PTWR owing to reasons including: the patient being absent at the bedside; the consultant starting the PTWR before 08:00; the pharmacist missing the PTWR owing to more urgent and complex medication history requiring pharmacist input; or ordering of non-ward stock items from the pharmacy being required for other patients. The pharmacist reviewed these patients after the PTWR; however, these contributions were outside of this service evaluation and were not included in the analysis.
A total of 549 non-classifiable exclusions (see Table 3) and 390 classifiable contributions (see Table 4) were recorded. On average, 1.6 classifiable contributions were made per patient seen on the PTWR by a pharmacist. A total of 1,753 active prescriptions were screened by pharmacists across PTWRs undertaken over the ten days.
| Table 3. Excluded non-classifiable contributions, with rationale | |
|---|---|
| Exclusion | Rationale |
| The number of complete prescriptions ordered (prescribed) on Cerner PowerChart, a prescribing system, by the pharmacist under the ‘prescriber contacted’ function or as an independent prescriber. | The number of medicines prescribed were excluded from the classifiable contributions as the total quantity would affect the scale of the overall contributions; therefore, a decision to analyse them independently was made. The total number of medications prescribed (n=246). |
| Laboratory/observations checked for the patient. This includes renal function, liver function, coagulation and bedside monitoring parameters. | It was agreed by the team through consensus that this vital check was made for each patient seen on post-take ward rounds (PTWRs) (n=247). |
| Ordering patient-specific medication from inpatient pharmacy and completing discharges on PTWRs. | The non-stock medication was ordered on the ward and is standard practice. Many discharges are written after the ward round (n=56). |
| Management and delegation responsibilities with medicines management technicians and preregistration pharmacists while on the PTWR. | Data were not collected; however, this is an acknowledged duty of the pharmacist (not counted). |
Contributions analysis
Table 4 shows the range of contributions and the numerical breakdown of each category (based on a previously published trust audit, to allow comparisons to be made with future audits and service evaluations)[18]
.
| Table 4. The number and type of classifiable contributions made by pharmacists on post-take ward rounds | |
|---|---|
| Reason for contribution | Number (%) |
| Clarification of intention | 60 (15) |
| Dose | 48 (12) |
| Venous thromboembolism risk assessment form prompted for completion | 40 (10) |
| Medication history/reconciliation issues | 38 (10) |
| Drug monitoring | 31 (8) |
| No medication prescribed for indication | 29 (7) |
| Adverse effect/interaction identified | 28 (7) |
| Unnecessary medication use | 28 (7) |
| Advice/recommendations provided | 19 (5) |
| Prescription duplication | 15 (4) |
| Drug selection | 15 (4) |
| Route of administration | 14 (4) |
| Other | 11 (3) |
| Frequency/time | 11 (3) |
| Formulation | 3 (1) |
| Total | 390 (100) |
Type, incidence and severity of errors
The panel rated 239 individual contributions (defined above), which examined the severity of the medication error (if any; see Figure 1).
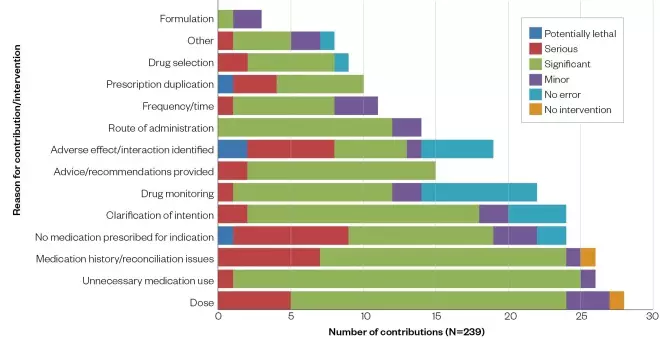
Figure 1: Type, incidence and severity of errors, which required a contribution on the post-take ward round as rated by the panel members
Of the 239 contributions or interventions made, the dose of the medication was the area in which most interventions were made. This ranged from under-dosing of antimicrobials to prescribed doses being higher than recommended.
Medicines reconciliation issues are often clarified by the pharmacist on ward rounds, and this was reflected in the results above, making up 11% of the overall contributions and interventions. As expected, contributions that had not resulted from error were reported. These were most commonly associated with drug monitoring, where monitoring of aminoglycosides was recorded on the ward round plan in response to the pharmacist’s contribution. Advice and recommendations provided were reported by the pharmacists; however, it was not recorded whether recommendations were followed by the medical team, so this was not analysed within this service evaluation.
‘Other contributions’ included referring the PTWR team to liaise with the renal team when a patient was admitted with high potassium and hypertension on dialysis, but was on ramipril prior to admission, so collaboration was needed to determine a safer alternative. Referrals to other teams for expert opinions, such as the microbiology team, link together wider MDT working. Interacting with patients, for example by taking drug histories, was also included in this category. The panel deemed almost two-thirds (n=151) of contributions to be significant.
Examples of ‘potentially lethal’ events/errors include:
- The prescribing of ceftriaxone 2g once daily to cover central nervous system infection/meningitis. This dose was subsequently changed to 2g twice daily, as per guidelines, on the pharmacist intervention.
- The dose of tacrolimus was prescribed as 20mg once daily; subsequently, the patient was flagged as a high priority drug history for the pharmacy technician. The confirmed daily dose was 2mg, and was changed on the ward round on the pharmacist intervention.
- Another patient was on community methadone identified by the pharmacist, but this was not mentioned in medical clerking. The patient was regularly on 60mg daily, so the risk of withdrawal, and the risks of re-initiation of methadone after more than four days of missed doses in the community, were identified. The intervening pharmacist advised on withdrawal management, as per trust policy.
Value of the pharmacist

Figure 2: The value of the pharmacist for each contribution on post-take ward round as rated by the panel members
Figure 2 shows the value of a pharmacist for each contribution, as rated by the panel. More than two-thirds (68%) of contributions were deemed to be significant or very significant. None of the contributions were thought to be inappropriate in nature.
Analysis of the data showed a strong correlation between the significant and serious errors, and the positive significance of a pharmacist in those events. A wide range of examples demonstrated this, including a serious drug–drug interaction between tacrolimus and clarithromycin, where the outcome was that the pharmacist spoke to the renal team to discuss an alternative atypical cover. The severity of the error/event was deemed serious and the value of the pharmacist was rated as very significant.
In another example, a patient was prescribed intravenous hydrocortisone, alongside oral prednisolone. As the patient was tolerating the oral dose, a decision was made was to remove the intravenous hydrocortisone. The severity of this therapy duplication was recorded as ‘significant’ and the value of the pharmacist in this scenario was rated as ‘very significant’.
Another case involved a patient who had been prescribed a statin that was contraindicated, which resulted in a rise of creatine kinase to 1966IU/L. A decision was made to withhold the statin. The severity of this event was serious, and the value of the pharmacist was considered to be significant. The pharmacist’s input on ward rounds is evidently valued, judging by the panel’s rating of the contributions. As earlier mentioned, the severity of the error (if any) and value of a pharmacist was based on the risk of harm if the error were to persist without intervention.
Discussion
From this service evaluation, we were able to determine the type of contributions being made (average number of contributions made per patient was 1.6) and the associated severity of the errors leading to a contribution. When the PTWR pharmacist has contributed to patient care through their interaction on a ward round, the largest contribution was ‘clarification of intention’ seen in Table 4. This plays an important part in facilitating the movement of patients through the hospital and easing bed pressures as it can help pre-empt discharge plans, especially through earlier ordering of medicines for discharge. Despite clarification of the plan being exampled as ‘no error’ within the published scale, verbal feedback from the panel stated that many of the contributions arose as a result of essential information being omitted by prescribers and, therefore, were deemed as errors. Cardiac and infection medicines had the most interventions, possibly owing to the titratable nature of the drugs and the drive for antimicrobial stewardship, as per local and national policies, respectively. Similarly, the prompting to assess VTE risk forms part of a national drive to prevent avoidable harm.
Dosing changes were the second largest area where contributions were made, which is in line with data collected in other similar settings[3]
. Cerner PowerCharts, where preconfigured dosing regimens are presented as part of the electronic system, would, in theory, help reduce this. Furthermore, electronic systems provide legibility, a clear audit trail and have reduced storage requirements. However, it has been recommended to collect data on local errors and to publish them to aid training of service providers, which in turn would help to raise awareness of the risks associated with electronic prescribing[19]
.
The effect of electronic prescribing on communication was studied at a large teaching hospital in England in 2019. The study highlights a rise in non-face-to-face communication as a potential result of more screen time on laptops and computers[20]
. Interestingly, it was noted from interviews with healthcare professionals, that, overall, communication using alternative methods focused more on problem solving. It was found that each patient interaction involved a more targeted discussion around specific medications[20]
. Although fewer patients were seen, more time was spent with each patient. The significance of this was not established, but patient interaction is a fundamental part of a pharmacist’s role in the hospital[20]
.
From the data generated in this evaluation, two-thirds of errors that required intervention were deemed to be ‘significant’ to ‘potentially lethal’. In practice, the more errors that are prevented, the better the service is deemed[15]
.
Several studies have evaluated the impact of errors and associated adverse drug reactions on average lengths of patient stays, including the cost implications for the NHS[21],[22]
.
None of the contributions were deemed to be inappropriate or present a poorer outcome for the patient; root causes are often determined on the event of a serious incident occurring. For instance, preventing anaphylaxis through early identification of allergy status may only be noted after the significant anaphylactic event has occurred.
Overall, two-thirds of contributions were deemed to be of significant value by the panel and efforts were made to reduce bias by involving an independent panel who assessed the contributions. Data were collected by a range of pharmacists with a full pharmacy team present, which mimics the daily service offered.
Limitations
The type of contributions made on an electronic prescribing system were not compared with prescriptions made on paper within this service evaluation[10]
. Also, the role of electronic prescribing has not been evaluated in this service evaluation; however, the literature reveiwed suggests that the rate of prescribing errors does not fall as expected and, in turn, other types of prescribing errors arise that are specific to the electronic system. The use of only electronic prescribing limits this service evaluation’s comparability to other similar studies in the past ten years.
Literature has established a link between the two ratings (the severity of medication errors and the ‘value’ of the intervention made by the pharmacist); however, the direct relationship has not been investigated in this evaluation. It can be extrapolated that the errors deemed ‘significant’ to ‘potentially lethal’ requiring a contribution from a pharmacist would be highly valued; however, without appropriate data analysis on causality links, this cannot be concluded in this article.
The panel were not presented with actual outcomes of the prescribing error, owing to the ethical issues of letting an error persist. This may have been because of reporter bias or prompting for an observational evaluation in the future.
Extremely significant contributions, such as making life-saving interventions, were not found. These interventions would largely encompass services such as cardiopulmonary resuscitation, or prescribing or administering medications, such as naloxone. This is a time-critical category and the pharmacists within this setting would deem it outside of their competency.
The time taken to collect data on the PTWR was deemed to be a limitation, questioning whether all contributions — accepted and rejected — were recorded. Owing to the fast-paced nature of the work, it could not be guaranteed that all clinical activity was captured, despite pre-data collection instructions being disseminated throughout the team.
If time had allowed, this data could have been presented to a variety of members of the MDT to help evaluate their perception of a pharmacist’s role based on the contributions presented to the panel.
Further work
This study was shared with the lead pharmacist for acute, emergency and elderly medicine; the executive lead pharmacist for research; the medical director for acute medicine; the acute and specialist medicine quality and safety meeting; and the pharmacy department at a lunchtime session.
The strengths and limitations discussed above were considered and the following recommendations were made, to help optimise and increase the efficiency of the service:
- Establish guidance for new pharmacists requiring PTWR training. This could be presented in the form of education sessions and the production of a learning proforma to standardise the approach on ward rounds between all grades of pharmacists;
- Develop an efficient handover system to enable follow-ups and outstanding work to be communicated to the downstream pharmacist. A proforma could be inserted in to the ‘medication history completed by pharmacy’ section on Cerner PowerCharts;
- Offer feedback to junior doctors in a protected but open environment, to help educate them about commonly occurring errors and the rationale behind the corrections. This can be carried out in scheduled weekly training sessions and, periodically, on induction days. Based on the panel review, examples classified under ‘clarification of intention’ could be fed back to doctors to ensure that timely decisions are made regarding prescriptions;
- Make sure that re-evaluation takes place as an observational study, because of presumed under-reporting and to measure improvement after the implementation of a standardised proforma tool.
A further improvement to the study would be to recruit a varied panel of experienced staff. Ratings would be based on personal experiences with the pharmacy team; personal experiences with errors and associated outcomes; and unintended bias from the guidance tool used in Table 1. Further investment is required in understanding errors and associated risk, to help standardise reporting of the significance of errors and likely outcomes, without the risk of bias, based on the level of experience and profession.
Following on from this evaluation, it is clear that value can be added to the service by increasing efficiency through training and communication with members of the MDT. The following action points were agreed upon:
- Present findings of this service evaluation to the pharmacy and medical team;
- Introduce standardised documentation (proforma) that the PTWR pharmacist is to complete for patients on Cerner PowerCharts;
- Use the proforma to train pharmacists on PTWRs, where it can be used to drive their thought process to increase efficiency;
- Carry out a re-evaluation after the implementation of the proforma and streamline the data collection process;
- Relay the findings at the acute and specialist medicine directorate governance meetings.
Implementing the use of a proforma on the PTWR
To aid training of new pharmacists on PTWR and efficient communication within the pharmacy team, a proforma was designed to prompt contributions (see Figure 3). Its design was based on the results from this service evaluation and aims to provide structure to the contributions. A mnemonic (A–G) was created to make the proforma short and memorable.

Figure 3: Proforma (A to G of post-take ward round) presented with a reconstruction of the ‘medication history confirmed by pharmacy’ section on Cerner and documentation pointer
Implementation of recommendations
The findings were presented to the acute medicine consultant, alongside the key learning points from the errors and contributions. There was a focus on high-risk medications, such as the timing of antimicrobials, anticoagulation and opioids. This was shared in the form of a teaching session at a Monday lunchtime education and training slot for junior doctors.
The PTWR proforma (Figure 3) was trialled as a prompting tool for two rotational pharmacists on induction. It was designed to be a quick reminder of the key interventions that could be made while screening on ward rounds. We concluded that the use of this tool would benefit those who found it difficult to structure their screening process on rounds. Established members of the team were already familiar with ward round processes and found that the tool might deflect from their method of working. Based on this, we made it optional to include the information in Figure 3 on each patient seen on ward rounds.
This service has not been re-evaluated since this service evaluation was carried out, but may be beneficial as staff rotate through the team.
Conclusion
This service evaluation provides an insight into pharmacists’ contributions on ward rounds and published methods of evaluating data. The presence of a pharmacist on PTWR has led to impactful interventions and should be used as a learning opportunity for the wider MDT. In addition, the contribution of pharmacists on PTWRs at ICHNT is highly valued, with their contributions leading to potential reduced harm. This ties in with the national work under way to ensure a greater proportion of pharmacist activities are on delivering clinical care to patients[23]
.
Financial and conflicts of interest disclosure
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. No writing assistance was utilised in the production of this manuscript.
Acknowledgements
With thanks to the emergency, acute and elderly medicine pharmacy team and physicians at Imperial College Healthcare NHS Trust.
About the author
Nickisha Patel is an emergency, acute and elderly medicine pharmacist, Patrick Foley is the lead emergency, acute and elderly medicine pharmacist and Seham Hussein Jama is the senior lead emergency, acute and elderly medicine pharmacist at Charing Cross Hospital, Imperial College Healthcare NHS Trust. Corresponding author email: nickisha.patel@nhs.net
References
[1] Fertleman M, Barnett N & Patel T. Improving medication management for patients: the effect of a pharmacist on post-admission ward rounds. BMJ Qual Saf 2005;14:207–211. doi: 10.1136/qshc.2004.011759
[2] Imperial College Healthcare NHS Trust. Quality account 2017/18. 2018. Available at: https://www.imperial.nhs.uk/about-us/who-we-are/publications (accessed October 2020)
[3] Bracey G, Miller G, Franklin B et al. The contribution of a pharmacy admissions service to patient care. Clin Med J 2008;8(1):53–57. doi: 10.7861/clinmedicine.8-1-53
[4] Cheong V, Tomlinson J, Khan S & Petty D. Medicines-related harm in the elderly post-hospital discharge. Prescriber 2019;30(1):29–34. Available at: https://www.prescriber.co.uk/article/medicines-related-harm-in-the-elderly-post-hospital-discharge/ (accessed October 2020)
[5] Mira J. Medication errors in the older people population. Expert Rev Clin Pharmacol 2019;12(6):491–494. doi: 10.1080/17512433.2019.1615442
[6] Bullock B, Donovan P, Mitchell C et al. The impact of a pharmacist on post-take ward round prescribing and medication appropriateness. Int J Clin Pharm 2019;41:65–73. doi: 10.1007/s11096-018-0775-9
[7] NHS England & NHS Improvement. Pharmacy and medicines optimisation: a toolkit for winter 2018/19. 2018. Available at: https://www.sps.nhs.uk/articles/pharmacy-and-medicines-optimisation-a-toolkit-for-winter-2018-19/ (accessed October 2020)
[8] Miller G, Franklin B & Jacklin A. Including pharmacists on consultant-led ward rounds: a prospective non-randomised controlled trial. Clin Med J 2011;11(4):312–316. doi: 10.7861/clinmedicine.11-4-312
[9] Bosma L, Jansman F, Franken A et al. Evaluation of pharmacist clinical interventions in a Dutch hospital setting. Pharm World Sci 2008;30:31–38. doi: 10.1007/s11096-007-9136-9
[10] Royal Pharmaceutical Society. Keeping patients safe when they transfer between care providers — getting the medicines right. Final report. 2012. Available at: https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Publications/Keeping%20patients%20safe%20transfer%20of%20care%20report.pdf (accessed October 2020)
[11] Kwan JL, Lo L, Sampson M & Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med 2013;158(5):397–403. doi: 10.7326/0003-4819-158-5-201303051-00006
[12] Connelly D. The Carter review: how are hospitals measuring up? Pharm J 2019;302(7921). doi: 10.1211/PJ.2019.20206050
[13] Brady D & Franklin BD. An evaluation of the contribution of the medical admissions pharmacist at a London teaching hospital. Int J Pharm Prac 2004;12(1):1–6. doi: 10.1211/0022357023213
[14] Dawoud D, Smyth M, Ashe J et al. Effectiveness and cost effectiveness of pharmacist input at the ward level: a systematic review and meta-analysis. Res Social Adm Pharm 2019;15(10):1212–1222. doi: 10.1016/j.sapharm.2018.10.006
[15] Overhage J & Lukes A. Practical, reliable, comprehensive method for characterizing pharmacists’ clinical activities. Am J Health Syst Pharm 1999;56(23):2444–2450. doi: 10.1093/ajhp/56.23.2444
[16] Dobrzanski S, Hammond I, Khan G & Holdsworth H. The nature of hospital prescribing errors. Brit J Clin Gov 2002;7(3):187–193. doi: 10.1108/14664100210438271
[17] Abdel-Qader D, Harper L, Cantrill J & Tully M. Pharmacistsʼ interventions in prescribing errors at hospital discharge. Drug Saf 2010;33:1027–1044. doi: 10.2165/11538310-000000000-00000
[18] Franklin B, Reynolds M, Shebl N et al. Prescribing errors in hospital inpatients: a three-centre study of their prevalence, types and causes. Postgrad Medi J 2011;87:739–745. doi: 10.1136/pgmj.2011.117879
[19] Franklin B & Puaar S. What is the impact of introducing inpatient electronic prescribing on prescribing errors? A naturalistic stepped wedge study in an English teaching hospital. Health Informatics J 2019; online. doi: 10.1177/1460458219833112
[20] McLeod M, Karampatakis G, Heyligen L et al. The impact of implementing a hospital electronic prescribing and administration system on clinical pharmacists’ activities: a mixed methods study. BMC Health Serv Res 2019;19(156). doi: 10.1186/s12913-019-3986-4
[21] Lada P & Delgado G. Documentation of pharmacists’ interventions in an emergency department and associated cost avoidance. Am J Health Syst Pharm 2007;64(1):63–68. doi: 10.2146/ajhp050213
[22] EEPRU. Prevalence and economic burden of medication errors in the NHS in England. 2019. Available at: http://www.eepru.org.uk/prevalence-and-economic-burden-of-medication-errors-in-the-nhs-in-england-2 (accessed October 2020)
[23] NHS. The NHS Long Term Plan. 2019. Available at: https://www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf (accessed October 2020)
