Introduction
Improvements in patient safety and healthcare delivery are increasingly important to clinicians. The 2008 UK National Confidential Enquiry into Patient Outcome and Death report “For better, for worse?” reviewed remediable factors in the process of care for patients who had died in hospital within 30 days of receiving systemic anticancer therapy (SACT). Part of the report focused on the prescribing and administration of SACT.[1]
The authors noted that handwritten cytotoxic prescriptions were of poor quality and, in their opinion, posed a considerable risk to patients’ safety.
There is limited published evidence around the implementation of electronic prescribing or to support the assumption that patient safety is improved by its introduction by reducing the number of prescribing errors.[2],[3],[4],[5]
The Department of Health and the National Chemotherapy Advisory Group have recommended that all SACT should be prescribed electronically where possible.[1],[6],[7],[8]
Chemotherapy prescribing is a complex process encompassing the prescription of cytotoxic medicines, infusion fluids and supportive care drugs, thus providing considerable potential for error. Cytotoxic medicines have a narrow therapeutic range and the actual dose is usually adjusted to body surface area (BSA), weight or creatinine clearance and, as such, minor alterations can have significant effects on cytoxic dose.
A medication error is described as an error that can occur in the processes of ordering, transcribing, dispensing, administration and monitoring of drugs.[3]
Before the introduction of computerised chemotherapy prescribing, drug errors were often attributed to illegible prescriptions and incorrect transcriptions of doses and medications.
The aim of this study was to identify the number and type of cytotoxic prescribing errors that occurred with computerised cytotoxic prescribing. This information was then compared with a similar audit (see Appendix) performed before electronic prescribing was introduced to assess whether or not computerised prescribing had reduced the frequency, or altered the type, of errors and improved patient safety.

Appendix
Methods
Setting
St Bartholomew’s is a central London teaching hospital and tertiary referral centre serving a local population of 1.2 million people. Approximately 11,000 prescriptions for cytotoxic medicines are generated each year between the departments of medical, clinical and haemato-oncology.
Current procedures
At present, all oral and intravenous chemotherapy at St Bartholomew’s Hospital is prescribed electronically using the ARIA (VARIAN) software system. This paperless form of prescribing holds over 400 chemotherapy regimens for different tumour types; all the parameters, except the cytotoxic dose, are preset by pharmacists and oncology consultants.
Cytotoxic doses are calculated based on patients’ BSA, weight or creatinine clearance. Baseline information, including diagnosis, stage, performance status, weight and height, as well as treatment intent and allergy status, is entered manually by the prescriber.
Doctors are granted prescribing rights following a period of training and written accreditation with the ARIA systems manager. Each prescriber must complete 20 prescriptions from varying regimens, which are then countersigned and approved by an accredited colleague (specialist registrar or above) and the senior pharmacist, to demonstrate competency before being permitted to prescribe independently.
Most prescriptions are issued before a patient’s attendance for treatment to ensure the pharmacy has sufficient time to prepare and dispense the chemotherapy. Therefore, more than one prescriber could be involved in the preparation and confirmation of a particular prescription in view of varying clinical commitments; prescriptions are subject to changes based on subsequent laboratory or clinical assessments.
An accredited oncology pharmacist screens chemotherapy prescriptions in advance of the day of treatment, and checks for errors as well as rechecking the most recent and relevant blood results. On the day of treatment, clinical staff confirm with the pharmacy that the chemotherapy should be prepared (this is done verbally or by an annotation on ARIA). The chemotherapy is then prepared and is checked by two pharmacists before being released to the ward/chemotherapy day unit.
Before chemotherapy is administered, two members of accredited nursing staff double-check the prescription, usually online or on a paper printout if mobile computer access is not available. All accredited nursing staff are able to view prescriptions and record the administration of drugs; however, they are not authorised to alter the prescription in any way.
All members of the multidisciplinary team with access to ARIA can annotate the clinical notes section, eg, to document a dose alteration or to record any concerns or queries from the patient.
Audit
The study was conducted over 44 working days between 17 November 2009 and 29 January 2010. A period of 14 days over Christmas was excluded in view of poor compliance with data collection.
Before the period of data collection, a data collection tool was developed and piloted by an oncology specialist registrar and a specialist oncology pharmacist. The data collection tool was used by pharmacists to document any identified errors, primarily at the point of screening. However, a form was also completed if an error was identified, for example, at ward level. Baseline information collected included:
- Patient identification number
- Chemotherapy regimen
- Whether the potential error was related to a chemotherapy regimen or supportive care (eg, prescription for granulocyte colony-stimulating factor)
- When and where the error was discovered (eg, in the pharmacy or on the ward)
- Whether the treatment was administered before discovery of the error
There was also a free text box where the pharmacist could clearly explain the error/potential error.
The information generated was reviewed on a weekly basis by an oncology specialist registrar and a specialist oncology pharmacist, and classified as:
- Actual error — a prescribing error that was dispensed and administered to the patient
- Potential error — a prescribing error that did not reach the patient but had the potential to impact on patient safety
Actual and potential errors were then classified according to clinical severity:
- Minor — no or negligible impact on patient care
- Moderate — some impact on patient care and safety
- Severe — serious impact on patient care and safety
For prescriptions that required subsequent alteration (eg, due to relevant abnormal haematological or biochemical results, or as a consequence of clinical assessment), the changes were classified as:
- Minor — <10% change in total chemotherapy dose
- Moderate — 10–20% change in total chemotherapy dose
- Severe — >20% change in total chemotherapy dose
This classification was used in combination with a clinical assessment of potential harm. For example, a >20% change in dose for a biologic therapy may be less harmful than the equivalent dose error for a severely myelosuppressive chemotherapy regimen.
The classification of the above errors was verified by senior consultants in both the medical oncology and haemato-oncology departments and also by the chemotherapy preparative senior manager.
The results of a previous audit, which was conducted in 2000 with the same criteria and preformatted paper prescriptions, were used as a historical control.
The Stata Version 8.2 software package was used to perform the statistical analysis. Differences in proportion were analysed using Fisher’s exact test.
Results
During the period of data collection 1,426 chemotherapy prescriptions were issued over 44 working days. Of these, 89 (6.24%) prescriptions were identified with at least one prescribing error (94 errors were identified in total; some prescriptions had more than one error). There were 79 prescriptions with errors involving SACT medicines, and 10 prescriptions with errors that related to medicines used for supportive care (see Box 1).
| Box 1: Errors identified, stratified by clinical severity | ||
|---|---|---|
| Severity of error | Number of prescriptions (% of all chemotherapy prescriptions) | Clinical example |
| Minor or no clinical significance | 42 (2.94) | Dexamethasone dose not reduced when aprepitant initiated |
| Moderate | 40 (2.8) | Documented adverse reaction to metoclopramide on ARIA — prescription issued without deleting metoclopramide |
| Severe | 7 (0.49) | 1,000-fold dose reduction of methotrexate |
| Total | 89 (6.24) |
For the purposes of determining clinical severity, the overall prescription was assessed for severity of error. There were 42 (2.94%) prescriptions that contained minor errors, 40 (2.8%) with moderate errors and seven (0.49%) with severe errors.
All but one error was identified at the point of screening; this prescription was dispensed but intercepted by the ward pharmacist. Of particular importance is the fact that no incorrect prescriptions were administered to any patient over this period. Therefore, all incorrect prescriptions were classified as potential errors.
The types of prescribing errors identified are outlined in Box 2.
| Box 2: Prescribing errors identified | |
|---|---|
| Type of error | Number |
| Dose amendment not prescribed as per protocol/notes | 17 |
| Other | 17 |
| Incorrect start date | 13 |
| Dose change due to blood results (urea, electrolytes and 10 creatinine) | 10 |
| Incomplete prescription | 10 |
| Incorrect adjustment of carboplatin dose by ARIA | 5 |
| Dose change due to blood results (full blood count) | 4 |
Patient allergy to prescribed product | 4 |
| Change in patient’s weight | 3 |
| Dose change due to other toxicities | 3 |
| Glomerular filtration rate calculation for carboplatin 2 incorrect | 2 |
| Incorrect cycle number | 2 |
| Incorrect dose capping/uncapping | 2 |
| Dose rounding | 1 |
| Inappropriate regimen prescribed | 1 |
These results contrast with an audit performed in 2000 (before the introduction of electronic prescribing of chemotherapy within the trust). At that time, 242 prescriptions (74%) had errors identified. Using Fishers exact test, the difference in proportion between the occurrence of severe errors in 2000 compared with 2010 was significant (4% [n=13] versus 0.49% [n=7], respectively; P <0.001). There was also a significant difference in the proportion of errors overall for prescriptions in 2000 compared with 2010 (74% [n=242] versus 6.24% [n=89], respectively; P <0.0001).
Of the errors in 2000, 196 (60%) were minor errors, 33 (10%) contained moderate errors and 13 prescriptions (4%) had severe errors (see Figure 1).
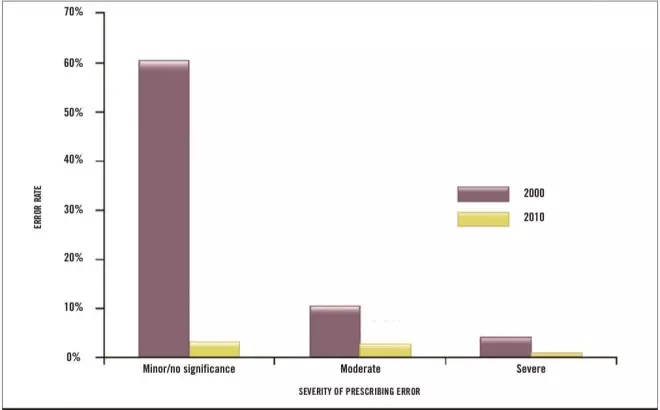
Figure 1: Comparison of the percentage and severity of prescribing errors before (2000) and after (2010) introduction of electronic prescribing
Discussion
Errors were identified in 89 (6.24%) of all electronic prescriptions. Forty-two (2.94%) had no potential to cause harm to patients, 40 (2.8%) could have caused moderate harm to patients, and seven (0.49%) were errors that could potentially cause serious harm to patients. The error rate was significantly decreased compared with an audit we conducted in 2000.
Our findings are consistent with two previous publications which showed a statistically significant reduction in the error rate noted when comparing electronically generated prescriptions with concurrent manual prescriptions.[3],[5]
An audit carried out at the University Hospital in Lausanne in Switzerland in 2006 compared the number of errors recorded before and after the introduction of computerised prescribing.[3]
Results showed that 15% (141/940) of manually prepared prescriptions contained errors, compared with only 5% (75/1,505) of electronically generated prescriptions. The authors believed that substantial improvement in patient safety was seen as a result of the introduction of computerised prescribing.
A prospective audit carried out in a haemato-oncology unit in Norwich also compared manual and electronically generated prescriptions — 20.4% of manual prescriptions had errors identified compared with 11.8% of electronic prescriptions. This represented a relative risk reduction of 42%.[2]
Error types also varied between both audits at our institution. Errors that occurred frequently with manual prescribing included incorrect transposition of doses and incorrect drug or infusion fluid prescribed. Information was also omitted from some orders, for example, the administration route, cycle number or patient details. Such errors were greatly reduced by electronic prescribing, with the most common remaining source of errors being dose amendment, due to the practice of pre-prescribing before the blood results were available. Huertas Fernandez et al demonstrated similar results in a paper published in the Journal of Clinical Translational Oncology in 2006.[2]
Their data also showed that the errors commonly detected with the use of manual prescriptions (such as the use of abbreviations and illegible handwriting) were eliminated by the introduction of the electronic prescribing.
We acknowledge that the intervening time between the two audits, as well as changes in practice, may have brought about changes in the error rate. Department of Health reports have recognised prescribing of cytotoxic medicines by junior doctors (senior house officer and below) as a potential source of error and, since the previous audit in 2000, senior house officers are no longer authorised to prescribe SACT and the training of all new prescribers was formalised as described in the methods.
Cytotoxic prescribing training as described was introduced with the implementation of e-prescribing, and has been compulsory for all trainees and consultants since 2006 to improve the quality of cytotoxic prescribing and reduce the error rate. In addition, following guidance issued by the National Patient Safety Agency in 2005, the chemotherapy preparation unit at St Bartholomew’s Hospital has introduced further safeguards to prevent errors occurring in the preparation and dispensing of chemotherapy. On the other hand, the increasing number and complexity of SACT protocols, the introduction of novel therapies and a growing number of prescribers might be expected to increase the risks of prescribing errors.
One of the most important benefits of the electronic record is as a communication tool for all the members of the medical, pharmacy and nursing teams to co-ordinate the safe delivery of SACT. However, this also highlights the continuing potential for errors to occur due to poor communication of intended dose changes, for example.
Our audit was performed in a single centre and we accept that the classification of errors is relatively subjective in the absence of any agreed standards.
The errors identified in this audit were multifactorial in origin and could be attributed to both human error and the manner in which the electronic prescribing system has been implemented in our institution. Two out of the seven severe errors identified were errors of dose adjustment. For example, a patient was prescribed mitomycin at a subsequent cycle despite documentation in the notes section of ARIA that the patient was not to continue the drug due to profound neutropenia. Currently we have to rely upon prescribers to open and read the notes on ARIA. This introduces the possibility of human error, which could be eliminated by making reading the notes before prescribing an action required by the system, or by the system flagging such entries in the prescribing window.
It has been recognised in this audit that the number of errors can be reduced by improving the way we have implemented the software. For example, a few chemotherapy regimens were set up to require a manual calculation of dosage units by the prescriber. This allowed a serious error in one prescription — a 1,000-fold lower dose of methotrexate was prescribed despite the presence of clear protocol information. Humans are prone to making errors and it is widely acknowledged that that system design needs to be used to minimise the potential for errors to occur. It is clear that the software system is only as good as the prescriber using it and the manner in which it is embedded in a safety orientated process of checks and balances.
Clear and concise communication is critical to minimise the number of cytotoxic prescribing errors. Although the electronic prescribing system is clearly of significant benefit, it can be further improved by making the safety-critical recording and reviewing of data and communication entries an absolute requirement before SACT can be prescribed, dispensed and administered.
A particular feature of prescribing at any large cancer centre is the prescription of chemotherapy several days in advance of a patient’s attendance for treatment to enable the pharmacy to manage workload and ensure rapid delivery to the patient on the day. However, this has identified shortcomings in the ARIA software, which does not accommodate this readily and does not enable the reliable adoption of dose modifications on the day or treatment. The addition of an additional prescription status step would enable this to be overcome.
Many of the errors arose through poor prescribing practice despite the training and accreditation that we had introduced along with the e-prescribing. We are therefore introducing an annual revalidation check, to emphasise the importance of accuracy in SACT prescribing, and placing it on par with intrathecal treatment. In particular, we found that oral SACT was poorly prescribed, and was occasionally dispensed before blood results were available leading to the risk that subsequent adjustments were not made or recorded. In line the recommendations of the NPSA,[9]
oral and intravenous SACT should be treated with equal care and we have therefore reinforced the training so that oral chemotherapy is not dispensed without appropriate blood results being reviewed by the prescriber, or by a pharmacist, using a set of prespecified regimen- specific acceptable blood parameters.
The implementation of electronic prescribing using the ARIA software has enabled us to improve the training of prescribers and the process for the dispensing and administration of SACT. By reducing the frequency of prescribing errors we have reduced the risk to patient safety and identified future training needs and software developments.
About the authors
Fiona McCarthy is specialist registrar for medical oncology, Saadhiya Hussain is specialist oncology pharmacist, Christopher Watson is specialist oncology pharmacist, Melissa Phillips is specialist registrar for medical oncology and C J Gallagher is consultant medical oncologist, all at Barts Health NHS Trust.
Email: f.mccarthy@qmul.ac.uk
References
[1] National Confidential Enquiry into Patient Outcome and Death. For better, for worse? A review of the care of patients who died within 30 days of receiving systemic anticancer therapy. 2008. www.ncepod.org.uk/2008sact.htm (accessed 24 Febraury 2012).
[2] Huertas Fernandez M, Baena-Canada J, Martinez Bautista, et al. Impact of computerised chemotherapy prescriptions on the prevention of medication errors. Clinical and Translational Oncology 2006;8:821–5.
[3] Voeffray M, Pannatier A, Stupp R,et al. Effect of computerisation on the quality and safety of chemotherapy prescription. Quality and Safety in Health Care 2006;15:418–21.
[4] Stoner NS, Tanfield CJ, Talbot DC. Computerised prescribing of chemotherapy reduces errors. BMJ 1996;312:707.
[5] Small M, Barrett A, Price G, et al. The impact of computerised prescribing on error rate in a department of oncology/haematology. Journal of Oncology Pharmacy Practice 2008;14:181–7.
[6] National Cancer Action Team. National cancer peer review programme 2004–2007. National Report. London: National Cancer Action Team; June 2008.
[7] Richards M. Variations in usage of cancer drugs approved by NICE. London: Department of Health; June 2004.
[8] Department of Health. Chemotherapy services in England: ensuring quality and safety. August 2009. www.dh.gov.uk/en/Publicationsandstatistics/Publications/DH_104500 (accessed 24 February 2012).
[9] National Patient Safety Agency. Patient safety risks of incorrect dosing of oral anticancer medicines. January 2008. www.nrls.npsa.nhs.uk/resources (accessed 24 February 2012).