Mclean/Shutterstock.com
Open access article
The Royal Pharmaceutical Society has made this feature article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/resources/pharmacy-guides/wuhan-novel-coronavirus

Source: McLean/Shutterstock.com
Development of a safe and effective vaccine is widely seen as our only permanent exit strategy from the COVID-19 pandemic and the best chance of returning to something that resembles ‘normal life’. And with over 300 vaccine candidates and dozens of clinical trials taking place, researchers are working feverishly to make that happen.
The average time to develop a vaccine for widespread use is around ten years, but during the COVID-19 pandemic, vaccine developers have condensed this process into a matter of months. This has meant carrying out certain stages of vaccine development in parallel; for example, millions of doses of vaccine are already being manufactured by pharmaceutical companies ‘at-risk’, which means they are doing so without knowing whether the vaccine will be effective.
So far, the UK government has hedged its bets, placing orders for 340 million doses of different vaccines from six manufacturers. If they all prove to be successful, this would be enough to provide a dose to more than five times the UK population, but this outcome is unlikely.
Currently, these vaccines are at different stages of development and are from four different classes: two adenoviral vector vaccines, an mRNA vaccine, an inactivated whole virus vaccine and two protein adjuvant vaccines (see Figure). The government has also provided £41m in funding to support trials of another RNA vaccine being developed by Imperial College London.
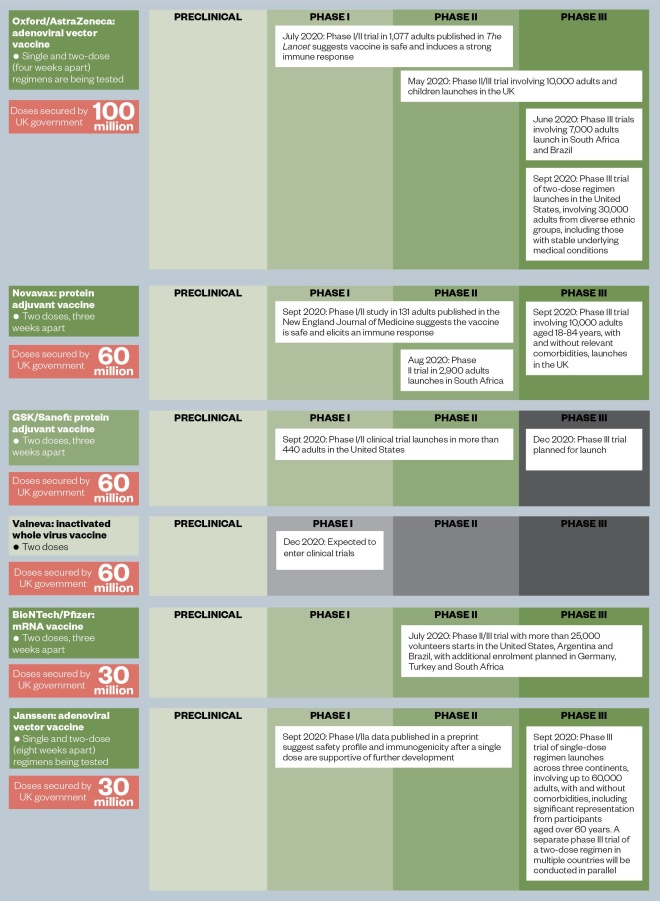
Figure: The UK vaccine portfolio
The UK government has secured access to a potential 340 million doses of six vaccine candidates, which are at various stages in the development process.
Source: World Health Organization, The Lancet, The New England Journal of Medicine, MedRxiv
The hope is that by taking so many different approaches, at least one will provide a useable vaccine to inoculate the UK population.
Matt Hancock, the health secretary, dramatically raised hopes in early September 2020, when he said that the UK would be “likely” to have a coronavirus vaccine by early 2021. But the reality is that there are still many hurdles to overcome even once a vaccine has been shown to be safe and effective, including creating sufficient manufacturing capacity and – perhaps the most difficult of all – ensuring public trust (see Box).
We must temper this optimism, this talk of the perfect vaccine ‘just around the corner’ or the idea that it will be a complete and immediate solution
And while the speed and scale of vaccine development has, so far, been extraordinary, there is also a need to manage expectations and to be cautiously optimistic about progress. Jeremy Farrar, director of the Wellcome Trust, said in September 2020: “We must temper this optimism, this talk of the perfect vaccine ‘just around the corner’ or the idea that it will be a complete and immediate solution”.
Before anyone can predict when we might have a useable vaccine against COVID-19, efficacy results from phase III trials are needed. If positive these need to be presented to the appropriate regulatory authority to authorise emergency use. Manufacturers then need to ensure there is enough vaccine in the freezer, ready to go, as well as the manpower and facilities to deliver it, fairly, to billions of people across the world.
Even then, the first generation of COVID-19 vaccines may only be partially effective — flu vaccinations, for example, are only 40–60% effective — not suitable for all population groups and only provide immunity for a limited period.
Box: How will a COVID-19 vaccination programme work?
Should any vaccine be found to be safe and effective, interim guidance from the Joint Committee on Vaccinations and Immunisations, which advises UK health departments on immunisation, says that care home residents and workers will be prioritised first, followed by those aged over 80 years, and health and social care workers.
Matt Hancock, the health secretary, has said that he expects pharmacists to have “a massive role” to play in administering vaccinations against COVID-19; and the government has proposed amendments to the human medicine regulations that would allow this to happen, such as expanding the scope of patient group directions to allow pharmacists to administer any medicine, including COVID-19 vaccines.
The government has also proposed a “new type of national protocol” to allow registered healthcare professionals who do not normally vaccinate, and people who are not registered healthcare professionals, to safely administer a licensed or temporarily authorised COVID-19 or influenza vaccine. This could mean that pharmacy technicians get involved in administering vaccines.
However, the success of any vaccination programme in achieving herd immunity is reliant on its uptake by the public. Experts have suggested that herd immunity to COVID-19 will only be achieved when approximately 60% of the population is immune, either through naturally acquired immunity after infection, or a vaccination.
Improving public confidence in the COVID-19 vaccine will be a challenge with the emergence of myths surrounding any potential vaccine. Research from University College London published on 23 September 2020, which involved over 70,000 UK participants, revealed that one in five people are unlikely to be vaccinated against COVID-19, with 30% of respondents having substantial beliefs that vaccines may cause future problems and around one in seven believing vaccines do not work[1]
.
Gino Martini, chief scientist at the Royal Pharmaceutical Society, says his concern is that, before COVID, “we had a problem with vaccine haters, but now there is a social media frenzy saying that the COVID-19 vaccine will be ‘untested’”.
“We need to stress to the public that ‘unlicensed’ does not mean ‘untested’,” he adds.
The frontrunner
The frontrunner within the UK portfolio is an adenoviral vector vaccine being developed at Oxford University with AstraZeneca. This technology has been around for several decades but a vaccine – against Ebola – has only recently been licensed for use in humans.
The team at Oxford was already working on a vaccine for Middle East Respiratory Syndrome (MERS) when COVID-19 emerged and was able to quickly adapt that technology to target SARS-CoV-2 (the virus that causes COVID-19), reaching phase III trials within months.
In contrast to some other adenoviral vaccines, which use human adenoviral vectors, the Oxford vaccine uses a genetically weakened version of a chimpanzee adenovirus to carry the genetic code for surface spike protein on SARS-CoV-2 into the cells of the body. Some people may already harbour antibodies to human adenoviral vectors, making the vaccine less effective, but antibodies to chimpanzee adenovirus are rare.
Results of the phase I trial for the Oxford vaccine, which were published in The Lancet on 20 July 2020, show that it is safe for use and induces an antibody and T cell response[2]
. It is now undergoing large phase III trials in the UK, South Africa and Brazil, with results anticipated before the end of 2020, depending on the rate of infection within the clinical trial communities.
The Oxford vaccine trial was paused in September 2020 after “a suspected adverse event” in a UK participant, but has now resumed in all countries except the United States.
The ‘plug in and play’ approach
For many vaccine developers, work began as soon as the genetic sequence of SARS-CoV-2 became available in January 2020.
This was the case for Janssen Pharmaceutical Companies of Johnson & Johnson, which – like Oxford University – is developing an adenoviral vector vaccine. In July 2020, the company received a marketing authorisation from the European Commission for its vaccine against Ebola, which uses the same ‘platform’ technology as its COVID-19 vaccine.
Jerome Custers, senior scientific director of vaccine research at Janssen, says this existing knowledge of the technology enabled a “sort of plug in and play” approach, which, like Oxford, has allowed researchers to develop the COVID-19 vaccine much more rapidly than if they were starting from scratch.
Janssen’s technology is based on a human adenovirus subtype called Ad26, to which neutralising antibodies are uncommon in Europe and the United States but more common in Sub-Saharan Africa.
In September 2020, Janssen launched a phase III trial of a one-dose regimen that will enrol up to 60,000 participants across three continents. It also plans to conduct a separate phase III clinical trial in multiple countries to explore a two-dose regimen.
“We have done a lot of preclinical work before we [moved] into clinical trials. For example, single immunisation with our vaccine was able to protect monkeys from a challenge with the SARS-CoV-2 virus,” explains Custers.
“Next to that we did a lot of characterisation studies in the laboratory and a lot of work on manufacturing of the vaccine to be able to manufacture at large scale.”
Custers says that, despite being familiar with the vaccine technology, because the virus is so new, his team had to start off relatively “blind”.
Along the way, massive amounts of information has become available, which we had to digest, implement immediately in our vaccine development efforts, and try to do everything in a very short timeframe
However, “along the way, massive amounts of information [has] become available, which we had to digest, implement immediately in our vaccine development efforts, and try to do everything in a very short timeframe”.
Janssen hopes to get approval for emergency use in early 2021.
The conventional approaches
Valneva is the only company in the UK government’s vaccine portfolio that has based its vaccine development on an approach that has been around for almost a century — an inactivated whole virus vaccine. Existing vaccines using this approach include those against hepatitis A and polio.
Valneva has already tested its technology for a variety of different viruses, from Zika to Chikungunya, enabling it to take a “copy paste approach”.
“This is also why we are very confident in meeting similar properties for our SARS-CoV-2 vaccine,” explains Thomas Lingelbach, chief executive of Valneva.
Valneva’s vaccine manufacturing site is based near Edinburgh, in Livingstone, and has received investment from the UK government to support its scale-up into a major UK vaccine facility.
The vaccine, Lingelbach says, is being produced “as we speak”. He explains that although vaccine development is still in the pre-clinical phase, which places it a long way behind some of the other developers, this is because of the nature of the technology.
“When you deal with a classical, whole virus technology, then you deal with the active live virus … before [inactivating] it chemically,” he says; this means labs and manufacturing facilities must be fully equipped to biological safety level three, the second highest standard in the world.
It took us three to four months to prepare our labs … so that’s the reason why we are a bit later than others; but, at the same time, we are working on a well-proven technology
“It took us three to four months to prepare our labs … so that’s the reason why we are a bit later than others. But, at the same time, we are working on a well-proven technology compared to many of the others.”
Valneva is taking an “innovative” spin on the classic technology by adding an approved adjuvant, CpG 1018, to improve the immune response invoked by the vaccine. The adjuvant, which is used in a hepatitis B vaccine, has been shown to be safe in the elderly, including the immunocompromised, which is a major target population for Valneva’s vaccine.
Having options like this available as part of the UK strategy will mean that the UK can decide which vaccine will be most suitable for each target population, Lingelbach explains.
“I am certainly convinced that there will be first-line defence vaccines — vaccines that you give to healthcare workers, so, healthy adults in [the] 18–55 [years] age range — but which you would not necessarily immediately give to special target populations.
“In the long run, I see our vaccine as a vaccine that covers [these] special patient populations.”
Valneva hopes to start clinical development of its vaccine in December 2020, with an anticipated conditional approval by the end of June 2021.
Another more conventional approach is protein-based vaccines, such as those already approved for influenza, human papillomavirus and hepatitis B. These vaccines do not require the same level of biological safety as live attenuated vaccines because they do not contain the pathogen or its genetic material, but they have been around for decades and are well understood, with a strong record of safety and effectiveness.
The most advanced protein-based vaccine candidate for COVID-19 is from Novavax, which has just kicked off a phase III trial involving 10,000 adults in the UK.
The UK government has secured access to 60 million doses of Novavax’s vaccine and a further 60 million of another protein-based vaccine being developed by GSK and Sanofi, which is currently in phase I/II trials.
These two vaccines both use recombinant versions of the spike protein and include adjuvants to boost the immune response.
The wildcard approach
Another technology in the UK portfolio is RNA vaccines, an innovative approach for which there are no vaccines currently licensed for human use. The UK has secured 30 million doses of an mRNA vaccine being developed by BioNTech and Pfizer, which is currently in phase III trials, and provided funding to support trials of another RNA vaccine being developed by Imperial College London.
Researchers at Imperial were already developing their RNA vaccine, funded by the Coalition for Epidemic Preparedness Innovations and UK Research and Innovation, but with a focus on Ebola, Marburg and Lassa viruses, rather than COVID-19.
The vaccine has the capacity to self-amplify within cells once it has been injected into the muscle.
“This potentially allows us to use lower doses and may induce a different quality of immune response with respect to the balance of antibodies and cellular immunity,” explains Robin Shattock, head of mucosal infection and immunity within the Department of Medicine at Imperial, and lead for its COVID-19 vaccine.
One challenge associated with this vaccine is that, as with all RNA vaccines, it will initially be a frozen product. “However, we will look to rapidly transition to a 2–8 degrees presentation with a freeze-dried product as a longer-term option,” Shattock says.
Currently, the vaccine is being tested in an expanded phase I study, which is aimed at optimising dose selection before moving to large-scale efficacy trials. Initially, the vaccine will be tested in adults up to the age of 75 years but, if successful, this will be expanded to include teenagers and children.
So far, the vaccine looks to be well tolerated and immunogenic, [but] we still need additional data before selecting the final dose to move forward
“So far, the vaccine looks to be well tolerated and immunogenic, [but] we still need additional data before selecting the final dose to move forward,” says Shattock.
As an academic lab, the team at Imperial does not have the financial reserves that large pharmaceutical companies can use to accelerate vaccine development timelines, and this has presented challenges.
“We have established a spinout company to commercialise along social enterprise principles and are in discussion with a number of manufacturers over global production,” explains Shattock.
“The other major challenge is establishing efficacy trials across international sites with limited funds, together with the numbers required to generate sufficient safety and efficacy data for approval,” he adds.
However, the team anticipates that positive data will be “transformative” and that, “if all goes well”, it could have regulatory approval by mid-2021.
Read more: Ten things pharmacists should know about COVID-19 vaccines
References
[1] Fancourt D, Bu F, Wan Mak H et al. Covid-19 social study results release 21. 2020. Available at: https://b6bdcb03-332c-4ff9-8b9d-28f9c957493a.filesusr.com/ugd/3d9db5_c2d0874d37b24bb5a9d535bf0e7f4f32.pdf (accessed October 2020)
[2] Folegatti P, Ewer K, Aley P et al. The Lancet 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4