View the full infographic here.
Drug approvals and market developments
Sildenafil showed promise as an oral treatment for erectile dysfunction (ED) in the early 1990s and was launched by Pfizer as Viagra in 1998. Since then three more PDE5 inhibitors have been launched in the UK.
1989: Sildenafil, a drug that selectively targets and powerfully inhibits the enzyme phosphodiesterase type 5 (PDE5), is first synthesised and tested in Pfizer’s UK laboratories.
1991: The first trial of sildenafil for coronary heart disease shows it is ineffective but penile erection is noticed as a side effect.
1998: Pivotal phase III trial of sildenafil for ED is published in The New England Journal of Medicine.
1998: Sildenafil (Viagra; Pfizer) is approved by the US Food and Drug Administration (FDA) in March and the European Medicines Agency (EMA) in September as the first oral treatment for men with ED.
2002: Tadalafil (Cialis; Eli Lilly) is approved for the treatment of ED by the EMA in November, and by the US FDA in November the following year.
2003: Vardenafil (Levitra; Bayer) is approved by the EMA for the treatment of ED in March and the US FDA in August.
2007: The European Commission approves low-dose (2.5mg and 5.0mg) tadalafil as single-daily ED therapy.
2008: Pfizer withdraws an application to the EMA to market Viagra over the counter for ED after the regulator expresses concerns that there would be no medical supervision, which could delay diagnosis of possible cardiovascular disease.
2009: Boots becomes the first UK pharmacy to offer Viagra without a prescription under a patient group direction.
2010: Tesco breaks into the Viagra market and offers the drug to customers without a prescription under a patient group direction.
2010: Vardenafil (10mg) is the first PDE5 inhibitor introduced for ED in an orodispersible tablet.
2012: Avanafil (Spedra; Vivus) is approved for treatment of ED by the US FDA in April and by the EMA in June 2013.
2013: The UK patent on Viagra expires, opening the way for generic versions of sildenafil to be launched.
2014: Manufacturer of Cialis, Lilly, agrees a deal with Sanofi to allow the French firm to buy exclusive rights to apply to sell Cialis as a non-prescription medicine in Europe, the United States, Canada and Australia after certain patents expire.
2014: Generic sildenafil can now be prescribed on the NHS for any male with ED, whereas previously it was restricted to those with ED because of an underlying health condition, such as diabetes or prostate cancer.
2017: The Medicines and Healthcare products Regulatory Agency (MHRA) recommends sildenafil 50mg (Pfizer) should be available as a pharmacy medicine, and launches a consultation that closes in April.
Usage and cost data for PDE5 inhibitors
The number of prescription items dispensed in England for the four PDE5 inhibitors has been influenced by their cost and restrictions on prescribing them within the NHS.
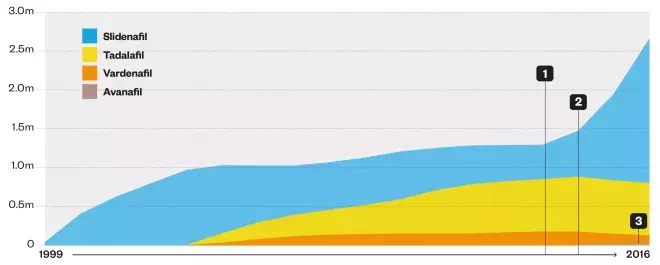
Number of prescription items dispensed in England
1. Introduction of low-price generic sildenafil and removal of prescribing restrictions led to a steep rise in the number of prescription items dispensed in England, from 1.3 million in 2012–2013 to 2.7 million in 2015–2016.
2. Branded tadalafil, vardenafil and avanafil, and Viagra, continue to be restricted on the NHS, so as prescriptions for sildenafil rose, those for tadalafil and vardenafil began to fall.
3. Avanafil (2015–2016): 6,765
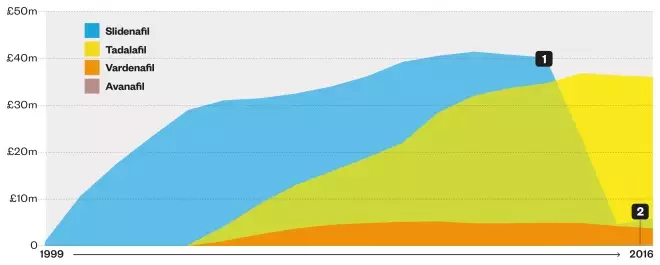
Net ingredient cost of prescription items dispensed
1. The net ingredient cost for sildenafil fell from £40.2m in 2012–2013 to £5.8m in 2015-2016, reflecting a fall in the cost per prescription item from £31.22 to £2.20
2. Avanafil (2015–2016): £148,113
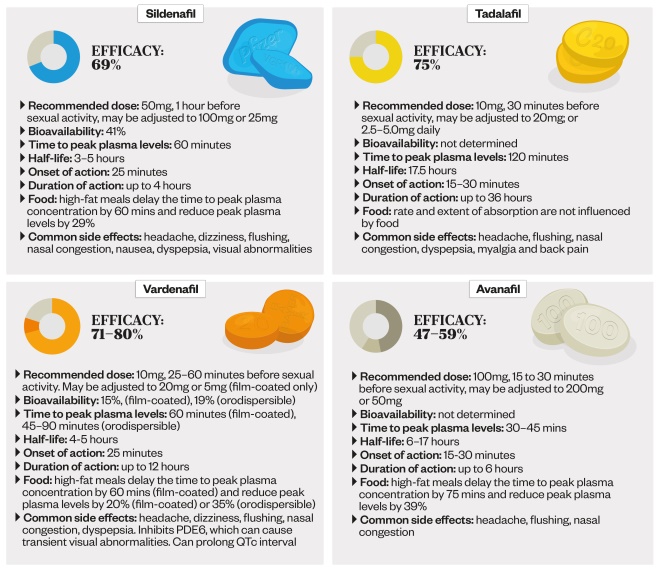
PDE5 inhibitors: key facts
PDE5 inhibitors work by increasing blood flow to the penis during sexual stimulation. Although all PDE5 inhibitors are effective, there are differences in their onset and duration of action. There are also differences in how their absorption is affected by food, and in their side effect profiles.
References
Sources: NHS Digital, MHRA, EMA, summaries of product characteristics, Pfizer, Bayer, Menarini
Editorial advisers: Ian Pearce, consultant urological surgeon, Central Manchester University Hospitals NHS Foundation Trust, and honorary senior lecturer, University of Manchester
Graphics: alisdairmacdonald.co.uk
You may also be interested in
