Abigail James
In 2016, after taking antidepressants for two years, patient James Moore realised that they were doing little to abate his emotional distress, so he visited his GP to ask about stopping them[1]
.
He is not alone. There is still debate around how effective antidepressants really are in daily clinical practice, despite performing better than placebos, on average, in randomised trials[2]
.
And more people are taking them: prescriptions in England alone have almost doubled over the past decade, rising to 70.9 million in 2018[3]
. These people need help to safely discontinue their treatment when it is ineffective.
Moore’s GP advised him to “take half a tablet [each day] for a week and then stop completely”, but these instructions had unpleasant consequences — he made repeated attempts to reduce his medication, but was left with “insomnia, nausea, panic attacks, dizziness, restlessness and agitation”, which his GP had not warned him about when he first prescribed the drug[1]
.
This experience was just a return of Moore’s original symptoms, he was told. After all, guidance at the time from the National Institute for Health and Care Excellence (NICE), originally published in 2009, stated that symptoms of withdrawing from antidepressants were usually “mild” and “self-limiting” over the course of a week or two[4]
,[5]
.
But Moore did not agree. His symptoms were new, more severe and variable[1]
. His feelings were echoed by the Royal College of Psychiatrists (RCPsych) which, in a position statement published in May 2019, said NICE’s recommended withdrawal period of four weeks could be too swift for patents who have been taking antidepressants for a long time, and who could face severe withdrawal symptoms for very long periods[6]
.
The National Institute for Health and Care Excellence’s guidelines ‘failed to acknowledge’ widespread withdrawal symptoms and ‘wrongly suggested that they usually resolve within one week’
RCPsych’s statement was based partly on evidence collated in a systematic review published in October 2018, which found that around 56% of people experience antidepressant withdrawal symptoms. And of those who have symptoms, nearly half (46%) describe them as severe[7]
. The review also suggested that long-term symptoms were not uncommon; 40% of people had symptoms lasting for 6 weeks, while for 25%, symptoms lasted 12 weeks or more[6]
,[7]
.
James Davies, reader in social anthropology and mental health at the University of Roehampton, and the lead author of the review, said that NICE’s guidelines “[failed] to acknowledge” widespread withdrawal symptoms and “wrongly [suggested] that they usually resolve within one week”[7]
. Like Moore, Davies worried that this lack of recognition “leads many doctors to misdiagnose withdrawal symptoms as relapse”, and unnecessarily and harmfully prescribe antidepressants for the long term[8]
.
Davies, along with RCPsych, called for clinical guidelines with a greater and more evidence-based focus on helping people to withdraw from their antidepressants. Then, in October 2019, NICE updated its guideline on depression to recognise the possible duration — “sometimes months or more” — and severity of withdrawal symptoms[4]
.
Guidelines recommend gradually reducing the dose of antidepressants, but there is still limited practical guidance for healthcare professionals and patients who want to do this
The guidelines still recommend gradually reducing, or tapering, the dose of antidepressants over a four-week period (although they recognise that some people may require longer). Despite this, there is still limited practical guidance for healthcare professionals and patients who want to do this.
However, there may be a simple solution that could make this difficult process easier, which is already used in some European countries, such as the Netherlands.
Lowering the dose each day
A factor making the tapering of medication difficult is the medication itself.
There are many products we use every day, such as shoes or prescription glasses, whose specifications are personalised to us.
However, when it comes to medicines, pharmaceutical companies obtain marketing authorisations for only a limited number of dosages. As medication comes in tablets or capsules of fixed dose, intermediate drug doses are not available, meaning it is very difficult to precisely define a tapering schedule in practice[9]
.
Some patients have employed their own makeshift solutions: using pill cutters and scales, counting granules from capsules, or switching to liquid forms and measuring cups. While these methods could work for those patients who are prepared to follow them, they prove too difficult for all who need them[9]
.
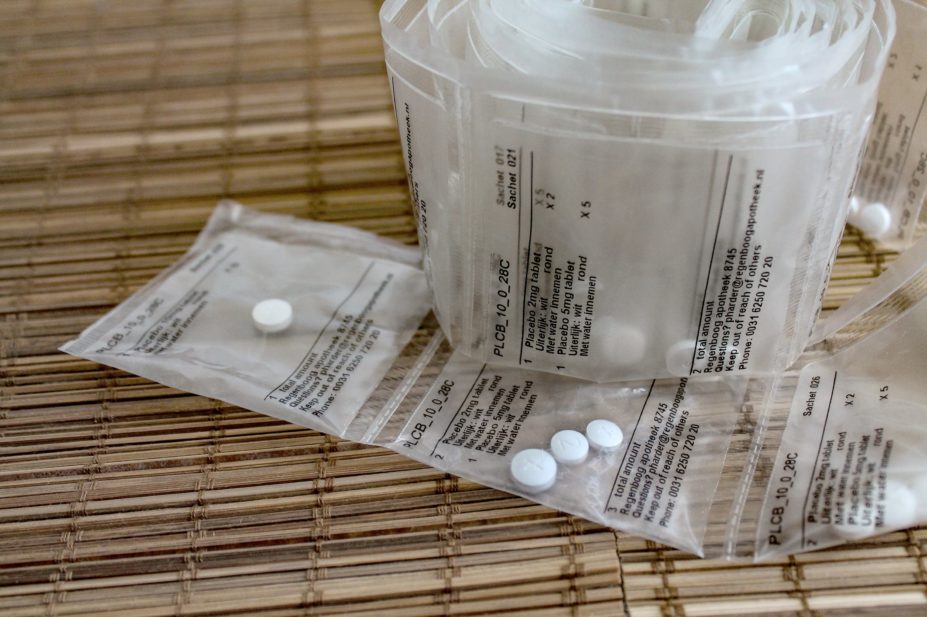
Tapering strips, which contain finely tapered doses to be taken each day, allow patients to regulate the speed of their dose reduction
However, tapering strips — a roll or strip of small daily pouches containing consecutively slightly lower doses to be taken each day — allow patients to regulate the speed of their dose reduction. This allows them to taper more gradually and conveniently than is possible with currently available medication forms[9]
.
Each strip covers a period of 28 days and the dose can be reduced over multiple strips. Information on dose and day, printed on each pouch, helps the patient to track and record their tapering progress[9]
.
Tapering strips are effective
Along with Jim van Os, professor of psychiatric epidemiology and public mental health at Utrecht University Medical Centre, I was involved in the first — and, to date, only — study to evaluate tapering strips. Conducted in the Netherlands, and published in May 2018, our observational study found them to be a simple and effective method for patients to achieve a gradual dose reduction[10]
.
Of the 1,194 users of tapering strips in the study, 895 (75%) wanted to discontinue taking antidepressants — most commonly paroxetine and venlafaxine. These patients had been taking these medicines for an average of two to five years[10]
.
Of 1,121 users who used the strips for tapering antidepressants (rather than for other medicines), nearly two-thirds (62%, n=692, although 97 were missing data) had tried to withdraw, unsuccessfully, at least once previously. Almost all of this 692 (97%, n=671) had experienced some degree of withdrawal (2–7 on a 7-point scale), while almost half of them (49%, n=339) had experienced the most severe degree of withdrawal (7 on the scale)[10]
.
Of the 895 patients who wanted to stop their treatment, 636 (71%) successfully tapered their medication completely, using a median of two tapering strips, over a median of eight weeks — double the duration of NICE’s four-week recommendation[10]
.
How tapering works
Abrupt cessation or too rapid a decrease in medicine can disrupt the body and cause withdrawal symptoms. Gradual reduction of selective serotonin reuptake inhibitors (SSRIs), for example, clearly reduces the risk of unwanted symptoms.
SSRIs work by inhibiting the serotonin transporter, and the relationship between the dose and the level of binding to the transporter is hyperbolic[11]
. When the patient is taking low doses, the level of binding increases very rapidly, and then levels off at higher doses[12]
. A patient using only registered doses takes steps down which are too large. When the patient begins tapering from a high dose, these steps have a limited impact on transporter binding. Bu, at low doses, this impact is significant.
To avoid withdrawal symptoms, dose steps down that are lower than registered doses are required to achieve smaller steps — this is known as hyperbolic tapering
So, even a small dose decrease can have a big effect. This hyperbolic relationship explains why withdrawal symptoms are often most problematic towards the end of a taper[12]
. Dose steps down that are lower than registered doses are required to achieve smaller steps to avoid withdrawal symptoms — this is known as hyperbolic tapering[13]
.
To picture how this process works, and to help patients understand the process, we can imagine a car that needs to reduce its speed to a complete stop from a very high speed.
Hitting the brakes a little harder at the beginning and then slowly releasing the pressure as the car slows down (also known as hyperbolic braking), brings the car to a complete stop smoothly[12]
.
However, hitting the brakes hard at once in an emergency stop runs the risk of banging your head against the windscreen[12]
.
In other words, to taper responsibly is to taper hyperbolically, for the entire duration of the tapering process[12]
. To minimise the risk of withdrawal symptoms near the end of the process, patients can take very small steps down, or leave more days between each step.
Choosing a tapering schedule
Of course, each patient’s tapering schedule will be different — there is no one-size-fits-all approach.
In an ideal world, the patient and healthcare professional would always work together, with shared decision-making, to develop a personalised tapering schedule.
Tapering strips, which allow for very small increments in dose reduction, could help the pair develop a very patient-specific schedule, and allow them to easily adjust the schedule, where necessary; an easy-to-use self-monitoring form provided with each strip makes this easier[14]
.
Bringing tapering strips to the UK
In the Netherlands, tapering strips are available on prescription and patients based here are reimbursed by some health insurers, but not all. They can be sent to patients outside of the country in accordance with the recipient country’s legislation[9]
. But, if the UK truly wants to reduce the number of patients taking antidepressants, it should make them available on the NHS.
Patient James Moore wrote to Matt Hancock, requesting him to trial tapering strips, with a view to implementing them across the NHS
Moore, who is based in Wales, used the strips to become drug-free after more than seven years of antidepressant use. In September 2019, Moore sent a letter, co-signed by experts, to the UK health and social care secretary, Matt Hancock, requesting him to trial tapering strips in a sample of general practices and psychiatric clinics, with a view to implementing them across the NHS[15]
.
Wendy Burn, president of RCPsych, supported this call for a trial in a separate letter[16]
. Moore, Burn and I have had positive discussions, but, as yet, there has been no response from Hancock.
But we must not leave patients in the dark; we need a nationally recognised tool that empowers everyone who wants to reduce their reliance on antidepressants to do so as gradually, safely and efficiently as possible.
Peter Groot, researcher in mental health patient experience, User Research Centre, Utrecht University, Netherlands; volunteer, Cinderella Therapeutics Foundation, a not-for-profit organisation.
Groot has no financial interests to declare.
References
[1] Moore J. 2019. Available at: http://www.letstalkwithdrawal.com/about-me/ (accessed April 2020)
[2] Guy A, Davies J & Rizq R (eds). 2020. Available at: https://prescribeddrug.info/ (accessed April 2020)
[3] NHS Digital. 2019. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018 (accessed April 2020)
[4] National Institute for Health and Care Excellence. 2019. Available at: https://www.nice.org.uk/guidance/cg90 (accessed April 2020)
[5] Robinson J. Pharm J 2019;303(7930). doi: 10.1211/PJ.2019.20207226
[6] Royal College of Psychiatrists. 2019. Available at: https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/position-statements/ps04_19—antidepressants-and-depression.pdf?sfvrsn=ddea9473_5 (accessed April 2020)
[7] Davies J & Read J. Addict Behav 2019;97:111Â−112. doi: 10.1016/j.addbeh.2018.08.027
[8] Robinson J. Pharm J 2018;301(7918). doi: 10.1211/PJ.2018.20205540
[9] Tapering Strip. 2017. Available at: https://www.taperingstrip.org/taperingstrips/ (accessed April 2020)
[10] Groot PC & van Os J. Psychosis 2018;10(2):142Â−145. doi: 10.1080/17522439.2018.1469163
[11] Meyer JH, Wilson AA, Sagrati S et al. Am J Psychiatry 2004;161(5):826−835. doi: 10.1176/appi.ajp.161.5.826
[12] Groot PC. 2019. Available at: https://www.pw.nl/achtergrond/2019/psychiatrische-patient-zeer-gebaat-bij-afbouwmedicatie
[13] Horowitz MA, Taylor D. Lancet Psychiatry 2019;6(6):538−546. doi: 10.1016/S2215-0366(19)30032-X
[14] Groot PC & van Os J. 2020. Available at: https://iipdw.org/wp-content/uploads/2020/01/PC-Groot-and-J-van-Os-User-knowledge-of-psychotropic-drug-withdrawal-Jan-2020.pdf (accessed April 2020) [manuscript under review]
[15] Moore J. 2019. Available at: http://www.letstalkwithdrawal.com/tackling-prescribed-drug-dependence-and-withdrawal/ (accessed April 2020)
[16] Bagot M. 2020. Available at: https://www.mirror.co.uk/science/people-taking-antidepressants-years-should-21538619 (accessed April 2020)


