REUTERS / Alamy Stock Photo
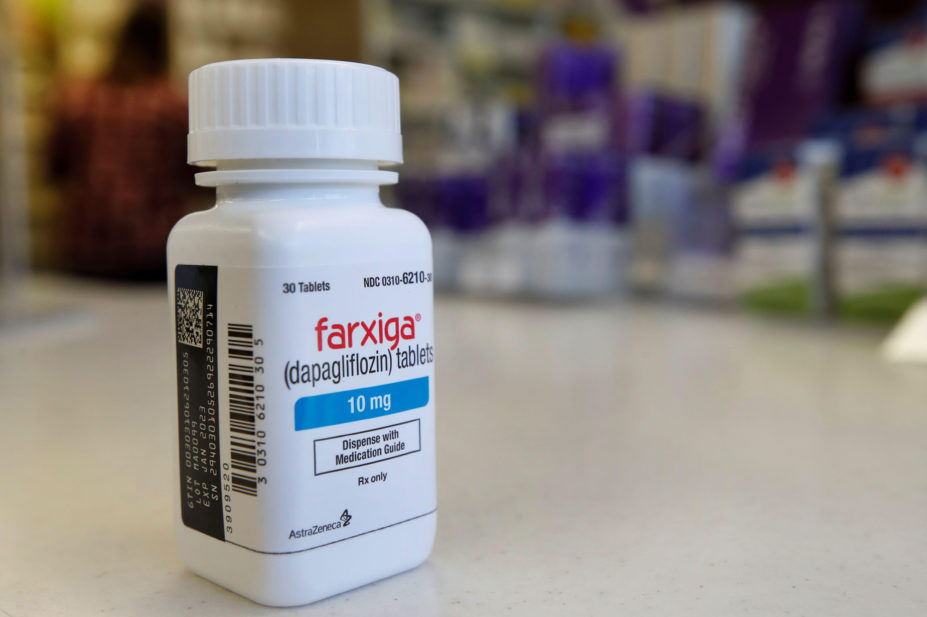
A decision by the Medicines and Healthcare products Regulatory Agency (MHRA) to grant a licence extension for dapagliflozin (Forxiga; AstraZeneca) for the treatment of chronic kidney disease (CKD) in adult patients has been hailed as a “significant milestone” in the treatment of the disease.
The decision follows promising results from the ‘Dapagliflozin and prevention of adverse outcomes in CKD’ (DAPA-CKD) trial, a phase III trial involving more than 4,000 patients with CKD stages 2–4 and elevated urinary albumin excretion, with and without type 2 diabetes mellitus (T2DM).
Trial participants were randomised to receive either 10mg of dapagliflozin — a sodium glucose cotransporter-2 (SGLT2) inhibitor — once daily or placebo, in addition to standard care, which included an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker. The primary outcome was a composite of a sustained decline in the estimated glomerular filtration rate of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes.
In addition to standard care, the researchers found that dapagliflozin significantly reduced the risk of kidney failure and prolonged survival in CKD patients with or without T2DM.
The licence extension means that dapagliflozin is now indicated for the treatment of adults with CKD. The SGLT2 inhibitor is already indicated for the treatment of symptomatic chronic heart failure with a reduced ejection fraction; and in adults for the treatment of insufficiently controlled T2DM, as an adjunct to diet and exercise.
David Wheeler, professor of kidney medicine at University College London, described the decision to extend the licence for the SGLT2 inhibitor as a “significant milestone” for people living with CKD.
“It provides healthcare professionals with an effective new option that can slow progression of disease and possibly delay the need for dialysis,” he said.
“As a clinician, I am thrilled to see advances like this, which have the potential to transform the experiences of many of the people living with this condition.”
Aaron Acquaye, renal pharmacist at Hull University Teaching Hospitals, said it was “welcome news” for clinicians and — most importantly — patients.
“The results of the study and subsequent licence extension adds one more tool to the arsenal to delay progression to end-stage renal disease; dapagliflozin was contraindicated in patients with a creatinine clearance of <60mL/min,” he explained.
“With the expected change in practice that will come as a result of this licence extension, pharmacists should remember to counsel patients started on dapagliflozin, or any SGLT-2 inhibitor, [on] the importance of maintaining adequate hydration and educate on how to recognise symptoms of diabetic ketoacidosis and what to do about it.”
In a press release issued on 8 August 2021, AstraZeneca said there are an estimated 3.5 million people in Great Britain with CKD and it is expected to become the fifth leading cause of mortality globally by 2040.