David Gee / Alamy Stock Photo
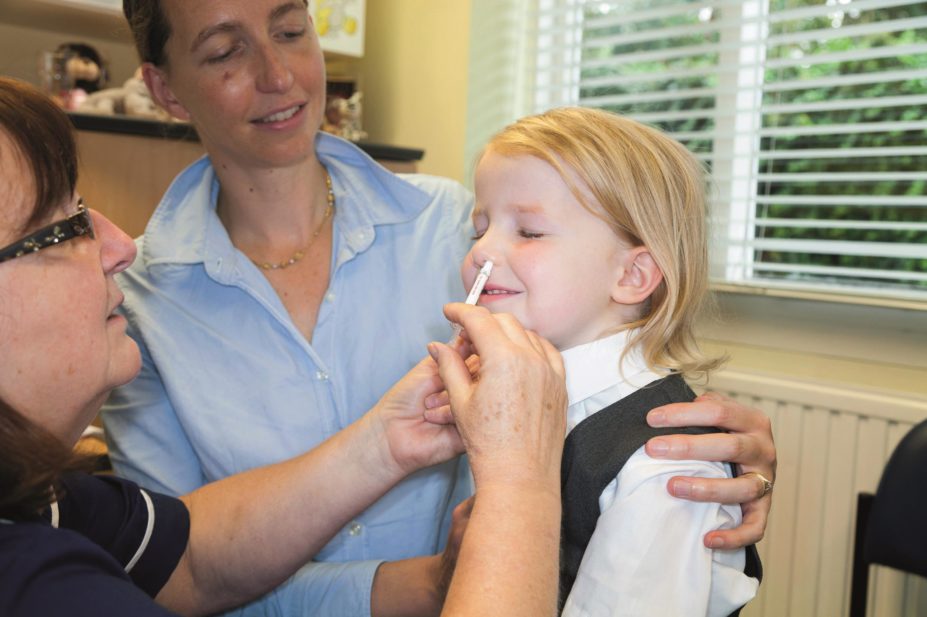
The nasal influenza (flu) vaccine used to vaccinate children aged 2–17 years in the UK achieved similar protection to adult vaccines in older age groups, according to provisional figures published by the Department of Health.
According to figures published by Public Health England (PHE) on 23 June 2016, the nasal vaccine is estimated to have been 57.6% effective (95% confidence interval [CI] 25.1–76) in preventing infection in children aged 2–17 years in the UK during winter 2015–2016.
The spray, which was made available under the national childhood immunisation programme in 2013, also had a knock-on effect on the number of adults presenting with flu when it was trialled in primary school children in England in 2014–2015, the figures show.
Consultation rates for adults with flu-like symptoms in some of the trial areas dropped by 59% compared with non-pilot areas, PHE says. There was also a 94% drop in GP consultation rates for flu-like illness in children within pilot areas, alongside a 74% fall in emergency department attendances for respiratory disease and a 93% decrease in hospital admissions for children with flu.
Richard Pebody, PHE’s head of flu surveillance, says: “These findings are encouraging and in line with what we also typically see for the adult flu vaccine. Based on intelligence to date, there is no reason to change current recommendations regarding use of the children’s nasal spray vaccine in the UK.”
The UK decision conflicts with the United States, where it was decided not to use the nasal spray after statistics from its Influenza Vaccine Effectiveness Network for the 2015–2016 season showed 3% effectiveness in children aged 2–17 years (CI -49% to 37%) for the inhaled vaccine, compared with 63% (CI 52% to 72%) with the injected vaccine.
You may also be interested in
Community pharmacies administered more than half of all COVID-19 vaccines to people from ethnic minorities, data show

NHS England seeks views on centralising flu vaccine procurement