Cristina Pedrazzani / Science Photo Library
The list, published by the DHSC on 23 August 2019.
Bedol (estradiol 2.0mg), Climanor (medroxyprogesterone acetate 5.0mg) and Clinorette (estradiol with norethisterone acetate 2.0mg and 1.0mg), manufactured by Resource Medical; FemSeven
Conti (estradiol with levonorgestrel 1.5mg and 0.525mg) and FemSeven
Sequi (estradiol with levonorgestrel 1.5mg and 1.5mg), manufactured by Theramex; and Cyclo–progynova (estradiol with norgestrel 2.0mg and 0.5mg), manufactured by Mylan, are now all “long-term out of stock”.
Resource Medical told The Pharmaceutical Journal
Zul
Akram, director of Resource Medical, said the company had been struggling for years to find a manufacturer to continue making the products for the UK market and had recently decided not to renew their Medicines and Healthcare products Regulatory Agency licence for these products.
“Of course we regret this, because the shortage is causing great difficulty to thousands of women at present, but the market is such that we were being under-prescribed in comparison to the bigger brands, such as Elleste [Mylan] and, therefore, wasting more than half of our stock.”
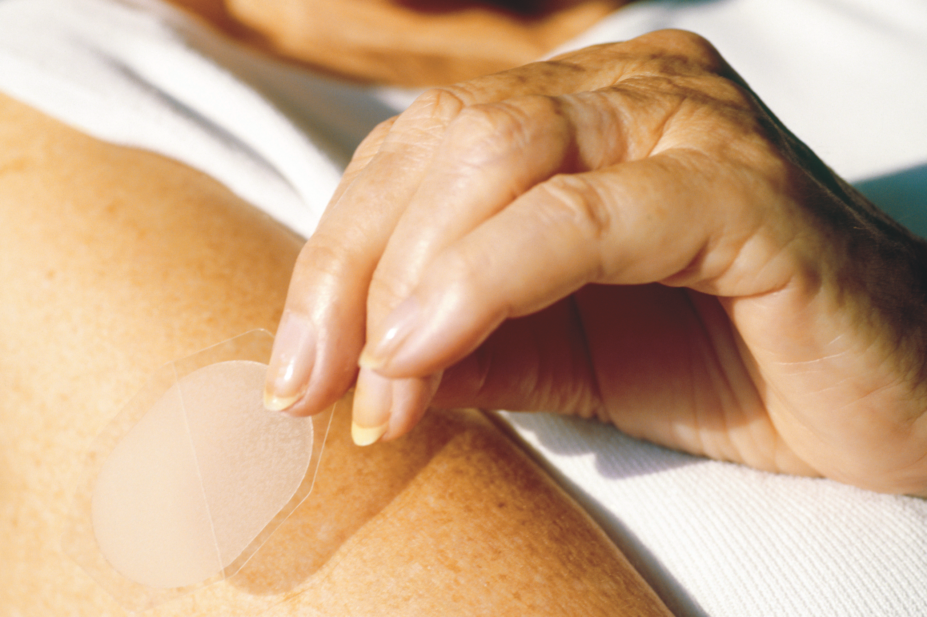
A spokesperson for Themex said the shortage of its FemSeven
Conti and Sequi products relates to quality issues with the adhesive that keeps the patches attached to the skin. They said that the products are unlikely to be back in stock until 2020.
“Currently, there is no other HRT with precisely the same composition as FemSeven
Conti and Sequi, and so we are unable to suggest any alternative treatments,” they said.
The list also highlighted that supplies of HRT products Evorel, Evorel
Conti, and Evorel
Sequi, manufactured by Janssen–Cilag, were in stock at the time of publication, but set to run out in September/October 2019.
A spokesperson for Janssen–Cilag said the company is experiencing “an unprecedented increase in demand for the Evorel range of products, due in part to the lack of availability of alternative HRT not produced by Janssen”.
An NHS memo
on Evorel
HRT patches, published by the Specialist Pharmacy Service on 22 August 2019, stated that an insufficient supply of transdermal
estradiol products, may mean “prioritising their use for women at increased risk of [venous thromboembolism]”.
DHSC guidance published alongside the list advised clinicians to “work closely with community pharmacies to understand local availability of HRT products” and use the list “to help make decisions about appropriative HRT products for patients who are affected by the supply issues”.
Ravi
Sharma, director for England at the Royal Pharmaceutical Society, said the information was useful for “contingency planning”, but would also reassure patients that there were alternatives available.
“Shortages happen all the time and pharmacists are doing an incredible job, going above and beyond to source people the right medicines.”
He added there would be regional variation in stock and encouraged pharmacists and clinicians to work locally to resolve shortages.
You may also be interested in
Government advises chief pharmacists to coordinate aid between trusts amid radioisotope shortage

Mandatory ‘eight-week buffer stock’ requirement not met by medicine suppliers, reveals audit
