Shutterstock.com
All glucagon-like peptide-1 receptor agonists (GLP-1 RAs) licensed for type 2 diabetes mellitus (T2DM) are facing a “national shortage”, the government has said.
A medicine supply notification issued by the Department of Health and Social Care on 27 June 2023 said the “high impact” shortage has been caused by “an increase in demand for these products for licensed and off-label indications”, with supply not expected to return to normal until at least mid-2024.
Until then, the notification said GLP-1 RAs should only be prescribed for their licensed indication, and clinicians should avoid initiating people with T2DM on GLP-1 RAs “for the duration of the national shortage”.
“Where there is reduced access to GLP-1 RAs, support people with type 2 diabetes to access structured education and weight management programmes where available,” it said.
“When prescribing an alternative class of glucose-lowering therapy, clinicians are advised to use medicines across the class evenly to mitigate the potential for further national shortages,” the notification added.
The government notification lists seven brands of four affected GLP-1 RAs (see Box), which are indicated for T2DM.
Esther Walden, deputy head of care at Diabetes UK, said the shortages are “disappointing” but welcomed the government guidance.
“While we understand that off-label prescribing can be beneficial in some circumstances, we cannot support it when it is directly contributing to ongoing shortages for those people living with type 2 diabetes and the impact this has on managing it well. We would encourage clinicians to be mindful of this impact and prioritise helping people to manage their diabetes,” she said.
“People living with type 2 diabetes should be reassured that there are a number of alternative treatments available to help manage their condition. Healthcare professionals should work with patients to find the best course of treatment for them.”
Speaking in a personal capacity, Claire Davies, a diabetes and endocrinology specialist pharmacist at Gateshead Health NHS Foundation Trust, told The Pharmaceutical Journal: “It is incredibly saddening that NHS and manufacturer supply chains have not been robust enough to prevent a large-scale shortage of a key medication for long-term condition management.
“GLP-1s are a well-recognised effective therapy recommended by the National Institute for Health and Care Excellence for management of type 2 diabetes for their ability to reduce HbA1c and weight. These supply problems are likely to cause uncertainty for many people living with type 2 diabetes.”
Davies added that the government’s guidance “should be commended for seeking clinical expertise from a range of multidisciplinary team members who work in the field of diabetes management to ensure clear guidance is available for clinicians dealing with these shortages”.
On 16 June 2023, semaglutide, dulaglutide, exenatide, and liraglutide were added to the government’s list of medicines that cannot be parallel exported from the UK or “hoarded”.
Box: Glucagon-like peptide-1 receptor agonists affected by supply issues
Semaglutide:
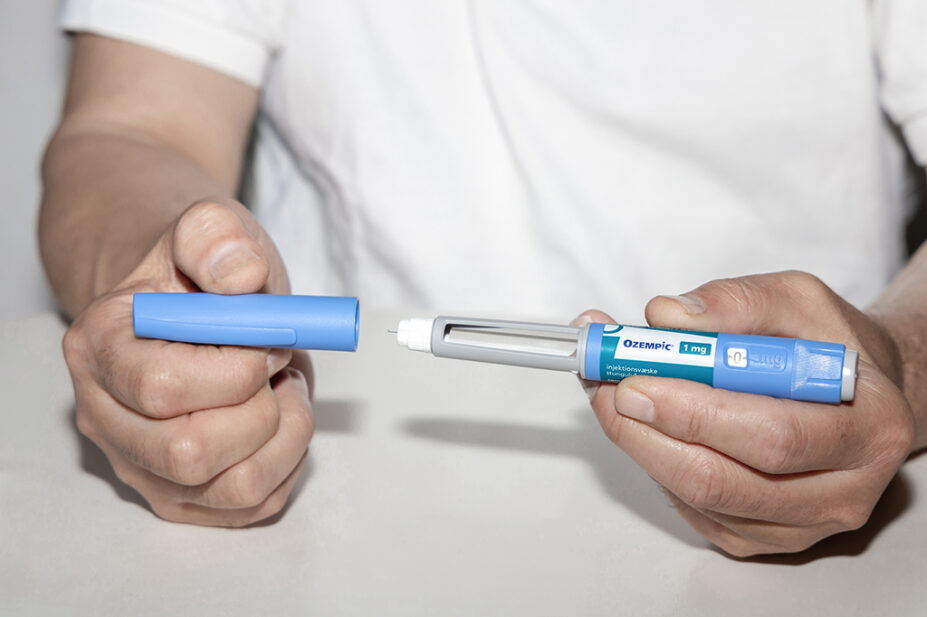
- Ozempic 0.25 mg solution for injection in pre-filled pen
- Ozempic 0.5mg solution for injection in pre-filled pen
- Ozempic 1mg solution for injection in pre-filled pen
- Rybelsus 3mg tablets
- Rybelsus 7mg tablets
- Rybelsus 14mg tablets
Dulaglutide:
- Trulicity 0.75 mg solution for injection in pre-filled pen
- Trulicity 1.5 mg solution for injection in pre-filled pen
- Trulicity 3 mg solution for injection in pre-filled pen
- Trulicity 4.5 mg solution for injection in pre-filled pen
Liraglutide:
- Victoza 6mg/mL solution for injection in pre-filled pen
- Saxenda 6mg/mL solution for injection in pre-filled pen
Exenatide:
- Byetta 5 micrograms/0.02mL solution for injection 1.2ml pre-filled pens
- Byetta 10 micrograms/0.04mL solution for injection 1.2ml pre-filled pens
- Bydureon 2mg/0.85mL prolonged-release suspension for injection 1.2mL pre-filled pens
You may also be interested in
Government advises chief pharmacists to coordinate aid between trusts amid radioisotope shortage

Mandatory ‘eight-week buffer stock’ requirement not met by medicine suppliers, reveals audit