When results from the ‘EV-302’ bladder cancer trial were presented at the European Society for Medical Oncology annual meeting in October 2023, they received a standing ovation. The study compared standard chemotherapy with enfortumab vedotin (Padcev; Astellas and Seagen) — a novel antibody drug conjugate (ADC) — given with the immunotherapy drug pembrolizumab in almost 900 people.
“It was the first standing ovation I ever saw at a large oncology meeting,” reports Dana Aftab, chief scientific officer at California-based cancer therapeutics company Exelixis. The results showed that the immunotherapy and ADC combination doubled survival time, with complete response rates increasing from 12% to 30%. The drug combination was approved by the UK’s Medicines and Healthcare Regulatory Agency (MHRA) in October 2024.
“It is the first time that bladder cancer patients may have the opportunity to face their disease without needing to take chemo[therapy] and deal with all of the horrible side effects associated with those older drugs,” says Aftab.
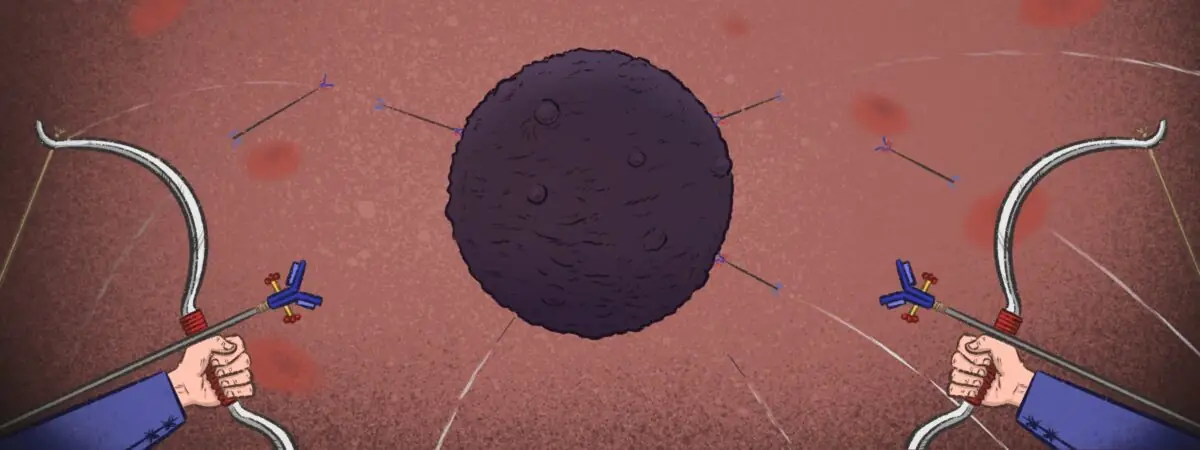
Padcev represents the next generation of ADCs — therapeutics that link a cancer-cell targeting monoclonal antibody (mAb) to a cytotoxic drug, known as the payload, via a chemical linker (see Figure). In the case of Padcev, the antibody targets the cell adhesion protein nectin-4, which is over-expressed in several cancers and carries a monomethyl auristatin E (MMAE) payload, a molecule that disrupts the function of microtubules, leading to cell death. Once bound, the conjugate is internalised and releases its toxic payload inside the cancer cell, without harming healthy cells. This targeting ability extends the therapeutic window, providing more cancer-killing ‘bang for your buck’.
As of mid-2024, 15 ADCs had been approved globally for clinical use, although progress has not been entirely smooth sailing. Gemtuzumab ozogamicin (Mylotarg), which was the first ADC to be approved in 2000 to treat acute myeloid leukaemia (AML), was withdrawn in 2010 after severe toxicity problems, but was reapproved at a lower dose in 2017. As recently as five to six years ago, the field “was struggling”, says Rob Noel, a biotechnology consultant and former business development director for contract development manufacturing organisation ADC Biotechnology.
“There were more [pharma companies] pulling out… and failures at phase III. And then, suddenly, Enhertu came through,” he says.
Enhertu (trastuzumab deruxtecan; Daiichi Sankyo and AstraZeneca) was first approved by the US Food and Drug Administration in 2019 and later by the MHRA in 2021 as a third-line treatment for advanced HER2-positive breast cancer. In 2022, the MHRA extended the licence to second-line treatment for advanced HER2-positive breast cancer and it is now also indicated for HER2-low breast cancer, specific forms of non-small cell lung cancer and gastric cancer.
The ADC uses the same targeting antibody found in the successful breast cancer ADC Kadcyla (trastuzumab emtansine) but carries a different payload and a different linker. Clinical trial results suggest Enhertu is superior to Kadcyla, with a progression-free survival of 28.8 months, 22.0 months longer than the 6.8 months offered by Kadcyla1. Trial results have also shown improved outcomes compared with chemotherapy in patients with low levels of HER22.
We’ve been talking about the potential of ADCs for a long time and I think we’re finally starting to see it play out
Penelope Drake, head of research and development, bioconjugates, Catalent Pharma Solutions
“We’ve been talking about the potential [of ADCs] for a long time and I think we’re finally starting to see it play out,” says Penelope Drake, head of research and development, bioconjugates, at ADC developers Catalent Pharma Solutions, based in California.
“There is a huge amount of investment going into [the] ADC [research & development] community,” adds David Spring, an expert in bioconjugation at the University of Cambridge, Cambridgeshire. Some of the companies that had terminated their ADC development programmes have jumped back in and there have been many acquisitions and licensing agreements. In 2023, Pfizer acquired ADC pioneers Seagen for around US$43bn and, in 2024, Johnson and Johnson acquired ADC developers Ambrx for approximately US$2bn, and Abbvie acquired ADC veteran Immunogen for US$10.1bn. Currently, the biggest players are Merck, AstraZeneca (in partnership with Daiichi Sankyo) and Gilead, which are sponsoring at least 33 phase III trials between them.
New payloads and linkers
There have been improvements since the first generation of ADCs. “Fifteen odd years ago, everyone wanted the most potent payload you could get, the most cytotoxic. But now it’s realised that, in fact, you don’t need that,” says medicinal chemist David Thurston, emeritus professor of drug discovery at King’s College London and the founder of several ADC companies. Enhertu’s payload, DXd, a topoisomerase I inhibitor that induces DNA damage, is a good example of a lower toxicity payload. “Now every company making ADCs wants to have something like DXd,” says Thurston.
Primary improvements seen in the newer generation of ADCs are in the linkers
Dana Aftab, chief scientific officer, Exelixis
However, according to Aftab, the “primary improvements” seen in the newer generation of ADCs “are in the linkers”. Changes to linker composition can improve drug stability, tumour cell permeability, and the number and sites of payload molecules carried by each antibody.
First-generation ADCs are produced using ‘non-selective conjugation chemistry’, whereby the payload is attached to the antibody via a maleimide linker molecule and neither the conjugation site nor the amount of drug conjugated to each antibody is controlled. But these types of linkers have limited stability, which means the toxic payload could be released prematurely.
Several new linker technologies have been developed, one of the first being ThioMab from Genentech, which modifies the antibody sequence itself to include cysteine residues in optimum positions that are then conjugated to produce homogeneous ADCs, with two payloads on each antibody3.
SmartTAG, another new linked technology developed by Catalent, encodes antibodies with an additional six amino acid tag. This allows the linker to conjugate via a much more stable carbon-carbon bond, avoiding early loss of the payload. “It’s got a lot of flexibility,” says Drake. “There’s leeway in where we can place that six amino acid sequence in the antibody, which means that we can intentionally design the location of the linker-payload.” This will help to make sure the ADCs are homogeneous and stable. The system is being used by Exelixis in its investigational ADC, XB010, which is currently in phase I trials to treat locally advanced or metastatic solid tumours.
Another approach comes from Dutch biotech Synaffix and makes use of the sugar molecules — called glycan — attached to antibodies. “Multiple studies have shown that the native glycan position is a privileged site for attaching ADC linker-payloads to antibodies,” says Floris van Delft, head of research and development at Synaffix. Its GlycoConnect technology modifies one of the antibody’s attached glycan using an enzyme followed by chemical attachment of the payload4. Synaffix announced licensing agreements with Boehringer Ingelheim and Mitsubishi Tanabe Pharma in early 2025.
One problem that all ADC producers face is their hydrophobicity — hydrophobic molecules are quickly cleared from the body by the liver, which decreases efficacy and increases toxicity. Usually, the more payload molecules added to an antibody, the more hydrophobic the ADC becomes, with the additional problem that individual ADCs tend to start clumping together, limiting solubility. “What you’re really driving for when you’re trying to optimise an ADC is limiting that kind of hydrophobicity,” says Drake, which can be achieved through innovations in linker design; for example, adding hydrophilic groups such as polyethylene glycol.
Cleavable linkers
Another innovation has been moving to ADCs that cleave their payload when they reach their target.
Initially, ADCs were designed to be non-cleavable with the whole conjugate internalised into a cell where the antibody is digested leaving the toxic payload and linker in the cell to act.
Everyone now wants a payload that will actually come out of the cell and kill neighbouring cells that are not carrying the biomarker
David Thurston, emeritus professor of drug discovery, King’s College London
“Often you see greater potency with a cleavable linker,” says Spring. They allow for payload release in the cancer micro-environment, unlike non-cleavable linkers that release the drug only inside the cell’s lysosome. One highly beneficial phenomenon noted with cleavable linkers is the bystander effect. Here, the payloads can kill ‘bystander’ cancer cells that do not carry the antibody target, either by release in the cancer micro-environment or from already targeted cells.
“Everyone now wants a payload that will actually come out of the cell and kill neighbouring cells that are not carrying the biomarker,” says Thurston. Drake thinks bystander activity explains much of the success of Enhertu.
Most ADCs are now being designed with cleavable linkers. The most common cleavage mechanism is via the protease cathepsin B, which is found inside cells and extracellularly around tumours.
“But a disadvantage is that these proteases could be in the circulation or in other tissues, causing the payload to release prematurely, before the ADC can get to the tumour. So you’ll sometimes get quite high levels of free payload in the circulation, which sometimes results in a lot of toxicity,” says Aftab.
There are more stable cleavage systems in the pipeline, including one designed by Spring, which uses an arylsulfate-group that can be cleaved by sulfatase enzymes inside cells that have low activity outside cells5. Spring has shown ADCs designed with these linkers to be more stable and also more soluble than some other systems, making them an attractive new solution.
Another innovative system from Catalent has a ‘double lock’ to prevent early cleavage. It is based on dipeptide cleavage but Catalent has added monosaccharide glucuronic acid, very close by, which physically blocks the protease and has the added advantage of making the molecule more hydrophilic. To release the payload, the sugar is removed using a glucuronidase — an enzyme — that is only active at the low pH found inside cell lysosomes and in tumour micro-environments, where the metabolic action of cancer cells creates a surplus of free protons6. Drake says animal studies are showing the linker provides improvements in tolerability and efficacy.
Side effects
Even with improvements from the first generation, all ADCs that are currently approved or in clinical trials have systemic toxicities. There will be some non-cancer cells that carry the biomarker and some off-target effects, which explains the number of failed clinical trials in the past year, including BioNTech and MediLink’s phase I trial for BNT326/YL202 in patients with non-small cell lung cancer (NSCLC) or breast cancer, which was halted in 2024 following multiple deaths. Aftab says the sort of peripheral neuropathy seen in chemotherapy can occur, particularly with MMAE payloads and can be dose limiting. But van Delft expects that, as pharmaceutical companies move to newer and more stable conjugation chemistries, this paradigm will shift, “with a reduction of the ‘traditional’ toxicities and shifting more into target-related toxicity”.
There are some other side effects specific to ADCs that can be problematic. In a process called micropinocytosis, ADCs can be taken into cells through small vesicles that form from the cell membrane. One of the results of this is occular toxicity. “Patients get dry eye, keratitis, even ulcerations. When it gets really bad in the eyes, they have to stop taking the drug,” says Aftab.
Another side effect — particularly with a topoisomerase I inhibitor payload — is interstitial lung disease, which is thought to be related to the presence of macrophages and other immune cells in the lungs that engulf large molecules like ADCs, leading to lung cell damage. “Those are the toxicities that, from now, we’re going to be working to try to avoid,” says Drake.
A clever strategy to target only cancer cells and limit side effects is conditional binding, which means generating an antibody that will only bind to the antigen when it is in the tumour micro-environment. Exelixis has licensed this technology from Chinese biotech Adagene, which has a SAFEbody platform that links a peptide to the antibody that covers the binding site and prevents the antibody from binding its target until it is in tumour tissue. Alternatively, some ADC developers are using pH dependent systems, in which the antibody will only fit the antigen binding domain at the low pH tumour microenvironment.
Despite the excitement around ADCs, there have been some high-profile failures in current ADC trials owing to a lack of efficacy, including Trodelvy (sacituzumab govitecan) from Gilead, for treating bladder cancer, and AstraZeneca and Daiichi Sankyo’s datopotamab deruxtecan (Dato-DXd), which failed to significantly improve overall survival in NSCLC patients . “The field is still trying to learn what the rules are,” says Drake, “there are a lot of improvements that can be made.”
What will happen next?
Although still at an early development stage, Catalent is pursuing bispecific antibodies that recognise two epitopes, either on the same target or on two different targets, doubling the chances of targeting a cancer cell. Already in a phase I trial, Sutro Biopharma has partnered with Merck on a bispecific ADC candidate, M1231, which includes a mucin 1 and epidermal growth factor receptor targeting antibody for treating advanced solid tumours. Catalent is also designing ADCs with dual payloads.
Others are looking at alternatives to antibodies as targeting systems, including antibody fragments, or even smaller peptides. “If it’s smaller, then it can penetrate tumour micro-environments better,” explains Spring. This could potentially also shorten the half-life of the drug to a few hours rather than weeks and might reduce the slow off-target release that causes toxicity. “We’ll see if there’s a significant benefit to them,” says Spring.
The ADC concept is also moving into other disease areas. Abbvie’s ABBV-3373 conjugates a small molecule payload to a glucocorticoid receptor targeting antibody and is currently in a first-in-human phase II trial to treat immune mediated inflammatory diseases7. However, a similar ADC for treating polymyalgia rheumatica and Crohn’s disease was discontinued in 2023, owing to its risk profile.
If you can target the antibiotic to the cells where you’ve got the infection, that will just increase that therapeutic index
David Spring, an expert in bioconjugation, University of Cambridge
Spring’s research group is using the approach to treat bacterial infections, such as antibiotic-resistant tuberculosis. A last resort antibiotic treatment, such as linezolid, can be toxic and lead to blindness if used for long periods. “If you can target it to the cells where you’ve got the infection, that will just increase that therapeutic index,” says Spring.
With large pharmaceutical companies onboard, the outlook seems positive for ADCs, but at what cost to the NHS and other healthcare systems? The UK list price for Enhertu, for example, is £1,455 per vial. United States list prices of ADCs currently on the market are in the region of US$2,000 (£1,600) to US$6,000 (£4,900) per vial.
Despite the excitement surrounding Enhertu’s performance, the National Institute for Health and Clinical Excellence (NICE) announced in November 2024 that it had failed to agree a price for it with AstraZeneca and Daiichi Sankyo, and the drug would not be available to patients with HER2-low breast cancer in England and Wales (although it is available in Scotland). Enhertu is temporarily available for eligible HER2-positive breast cancer patients via a managed access programme as part of the cancer drugs fund, and data from this will be used by NICE to make final recommendations for wider NHS use.
Noel says, although ADCs are drugs involving “an awful lot of chemistry”, the manufacture of each individual part follows well-established procedures. “The majority of the cost is in the raw materials,” he says and that will be impacted by the quantity needed. Initially it was thought that ADCs would be given at lower doses — micrograms per kilogram (of body weight) rather than milligram per kilogram, but currently, Noel says, “what’s interesting is their dosage isn’t that much lower than something like [the monoclonal antibody] Herceptin”.
From a development point of view, Drake says, right now “the goal is still to make the best drug you can make, and not to think as much about the potential costs”.
“We’re at this place where you’re starting to see more and more ADCs built with different designs in the clinic, and that’s going to help us learn what’s working,” she adds.
Aftab concludes that, so far, progress with the next generation of ADCs “is just the tip of the iceberg”.
- 1.Hurvitz SA, Hegg R, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. The Lancet. 2023;401(10371):105-117. doi:10.1016/s0140-6736(22)02420-5
- 2.Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan (T-DXd) versus treatment of physician’s choice (TPC) in patients (pts) with HER2-low unresectable and/or metastatic breast cancer (mBC): Results of DESTINY-Breast04, a randomized, phase 3 study. JCO. 2022;40(17_suppl):LBA3-LBA3. doi:10.1200/jco.2022.40.17_suppl.lba3
- 3.Junutula JR, Raab H, Clark S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925-932. doi:10.1038/nbt.1480
- 4.van Geel R, Wijdeven MA, Heesbeen R, et al. Chemoenzymatic Conjugation of Toxic Payloads to the Globally Conserved N-Glycan of Native mAbs Provides Homogeneous and Highly Efficacious Antibody–Drug Conjugates. Bioconjugate Chem. 2015;26(11):2233-2242. doi:10.1021/acs.bioconjchem.5b00224
- 5.Bargh JD, Walsh SJ, Isidro-Llobet A, Omarjee S, Carroll JS, Spring DR. Sulfatase-cleavable linkers for antibody-drug conjugates. Chem Sci. 2020;11(9):2375-2380. doi:10.1039/c9sc06410a
- 6.Chuprakov S, Ogunkoya AO, Barfield RM, et al. Tandem-Cleavage Linkers Improve the In Vivo Stability and Tolerability of Antibody–Drug Conjugates. Bioconjugate Chem. 2021;32(4):746-754. doi:10.1021/acs.bioconjchem.1c00029
- 7.D’Cunha R, Kupper H, Arikan D, et al. A first‐in‐human study of the novel immunology antibody–drug conjugate, ABBV‐3373, in healthy participants. Brit J Clinical Pharma. 2023;90(1):189-199. doi:10.1111/bcp.15888