This content was published in 2011. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Key points
- Warfarin has a delayed onset of action (maximum effect occurs up to 48 hours after administration) and prolonged effect.
- Target INR ranges depend on indication. INRs less than 1.5 increase risk of thrombosis and over 3.5 increase risk of intracranial haemorrhage.
- Patient-held records should be reviewed before dispensing warfarin.
Warfarin has been the main oral anticoagulant used for the prevention and treatment of venous thromboembolism (VTE) and embolic stroke for over 50 years. The expanded therapeutic role for anticoagulation and an ageing population has led to a rapid increase in patients prescribed warfarin and associated monitoring services over the past two decades.1
It is estimated that 840,000 people require long-term anticoagulant therapy in the UK. Increased national focus on addressing under-diagnosis of atrial fibrillation (AF) and ensuring appropriate antithrombotic therapy as part of the stroke prevention agenda suggests that the number requiring oral anticoagulation is set to increase further.
Regular international normalised ratio monitoring and appropriate warfarin dose adjustment coupled with patient adherence are important factors in achieving good anticoagulation control and preventing thrombotic and haemorrhagic events.
Pros and cons of warfarin
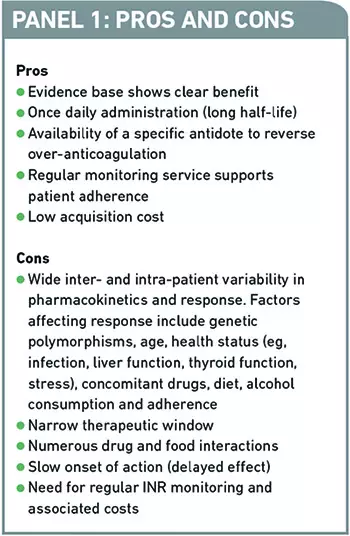
Studies have shown that warfarin significantly reduces the risk of primary and secondary VTE. For example, warfarin reduces the risk of ischaemic stroke by approximately 67 per cent in patients with AF.2 However, clinical and operational issues can make managing warfarin patients challenging. Panel 1 summarises the pros and cons of therapy.
In addition, warfarin continues to be under-prescribed because of clinician fears about the risk of bleeding and the perceived inconvenience of regular monitoring.3 Studies on clinicians’ perceptions suggest that they frequently underestimate the benefits and overestimate the risks of oral anticoagulation therapy.3 For instance, many patients are excluded from receiving oral anticoagulation if they have a history of falls because of a perceived increased risk of major bleed, but one study has estimated that a patient would need to fall around 295 times in one year (the reported mean rate of falls per year in the elderly was approximately 1.81) for the risk of intracranial bleed to outweigh the stroke prevention benefit of warfarin.4
Thus there is a need to dispel myths surrounding warfarin therapy through promoting a clear understanding of the evidence base and ensuring clinical governance is in place for effective anticoagulation services. The latter can be achieved through implementation and regular audit of the National Patient Safety Agency (NPSA) guidance on actions that make anticoagulants safer.5
Reflect
- How often should INR be monitored?
- How should patient-held records be reviewed?
- What are the alternatives to warfarin therapy?
Before reading on, think about how this article may help you to do your job better.
Warfarin initiation
Warfarin antagonises vitamin K, resulting in decreases in functional vitamin K dependent clotting factors. Initiating therapy is a fine balance between ensuring a therapeutic INR is reached in a timely manner to prevent thrombosis while avoiding excessive anticoagulation and haemorrhage. There is an increased risk of haemorrhage during the first four weeks of anticoagulation and such events can affect the subsequent acceptability of warfarin to both patients and clinicians.
An appreciation of the pharmacology, pharmacodynamics and pharmacokinetics of warfarin facilitates safe initiation. Warfarin has a long half-life of about 40 hours so it takes about eight days to reach steady state. To reduce the time taken, loading doses are frequently used. However, another key component to the onset of action of warfarin is the time taken for functional vitamin K dependent clotting factors to be depleted. The clearance of these clotting factors depends on their half-lives, which vary widely. At the start of warfarin therapy initial changes in INR are due to depletion of factor VII (shortest half-life, six hours). The actual antithrombotic effect of warfarin depends on the clearance of prothrombin, which has a half-life of between 50 and 72 hours. Therefore antithrombotic action is not typically present until day 5. Warfarin has both a delayed onset of action and prolonged effect; the maximum effect of a dose occurs up to 48 hours after administration and the effect can last five days. After discontinuation of warfarin dose, INR typically takes five days to return to normal.
Loading protocols
A variety of warfarin loading protocols are in use.7 The optimal protocol has been the subject of much debate over the past decade and two Cochrane reviews are currently under way to evaluate the most appropriate loading dose regimens in different indications. The two primary dosing strategies use either 10mg or 5mg as an initial dose. There is no evidence to suggest that a 10mg dose is superior to a 5mg initial dose, but consecutive high loading doses (eg, ≥10mg for two days or more) are associated with an increased risk of INR overshoot and haemorrhage.
Despite the range of protocols available there is general consensus as to when fast or slow initiation regimens would be most suitable. The British Committee for Standards in Haematology guidance recommends a slow regimen (eg, 2mg–5mg as initial dose) for outpatients who do not require rapid anticoagulation; therapeutic anticoagulation should be achieved within three or four weeks in most patients. For those requiring rapid anticoagulation because of high risk of imminent thrombosis (eg, acute pulmonary embolism), a faster regimen is more appropriate. In addition, co-prescribing a parenteral anticoagulant, such as a low molecular weight heparin (LMWH), is recommended to ensure an immediate anticoagulant effect is provided until the INR is stabilised. The parenteral anticoagulant should be continued for at least five days and until the INR is therapeutic for at least 24 hours, whichever is longer.6
Data have shown merit in using age-adjusted initiation regimens to account for the increased sensitivity of elderly patients to warfarin.6 Other patient groups that may benefit from a reduced initial dose include those with low body weight, hepatic impairment or cardiac failure.
Blood tests and targets
The INR is an expression of the time it takes for a fibrin clot to form (prothrombin time) following the addition of thromboplastin (a tissue extract containing tissue factor and phospholipids required to activate clotting). The results are adjusted to allow for the sensitivity of the thromboplastin used, making the INR test reproducible across the world. Essentially it is a measure of anticoagulation.
Because warfarin is dosed to INR effect, exhibits wide inter- and intra-patient variability and has a narrow therapeutic window, patients require frequent INR monitoring at the start of therapy. This enables timely assessment of the dose required to achieve and maintain a therapeutic INR.
The relative frequency of monitoring during initiation will depend on the indication and intensity of loading. Treatment for acute thrombosis often necessitates initial daily or alternate day monitoring, whereas for outpatients being slow loaded initial weekly monitoring may be appropriate. As consecutively stable INR readings are achieved intervals between monitoring appointments are gradually increased. The maximum recommended interval is 12 weeks. Most stable patients attend their clinic every eight to 12 weeks.
A growing evidence base over many years for the use of warfarin in a number of indications has led to widely accepted target INR ranges and durations of treatment to support appropriate management and dose adjustments. Examples are given in Panel 2.6
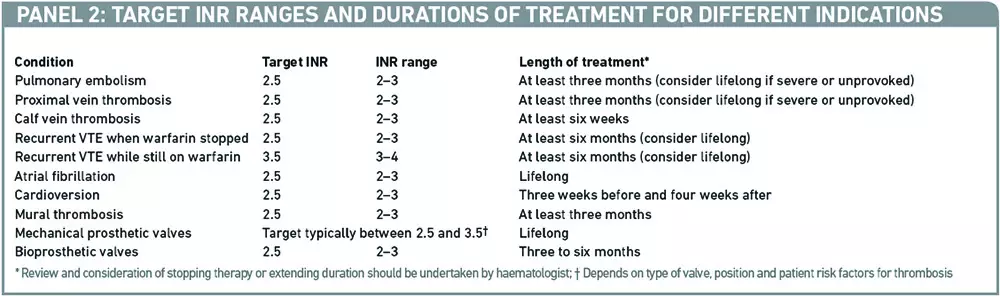
Any decision to deviate from the usual target range for a condition needs to be taken carefully, due to warfarin’s narrow therapeutic index. INRs less than 1.5 leave the patient at increased risk of thrombosis, while INRs over 3.5–4.0 increase the risk of intracranial haemorrhage. Occasionally, tight target ranges are specified (eg, 2.5–3.0 or 3.5–4.0) but this approach should be avoided because it results in more monitoring, an increased risk of thrombosis and bleeding and no benefit in terms of anticoagulation control.6
Testing options
Sampling can be venous or capillary. In venous sampling, blood is drawn from a vein into a sodium citrate collection tube. The quantity required depends on the brand of tube used but is in the order of 1.5–3ml. in the laboratory the sample is centrifuged and platelet poor plasma removed. Thromboplastin is added and the time taken for the sample to clot is measured optically.
In capillary sampling, a lancet is used to obtain about 10ml of whole blood. The blood is placed onto a disposable reagent strip within a coagulation point of care testing (POCT) device. INR results are typically available within 60 seconds. Although laboratory INR determination is still used by some anticoagulation clinics most now routinely use capillary sampling and POCT.
Some patients have purchased POCT devices (in the region of £400) and are self-monitoring INRs with advice for dose adjustments from their anticoagulation clinic. In addition, small numbers of patients have been supported to self-manage their anticoagulation; testing their INR and adjusting their warfarin doses themselves. They will usually be seen at least annually in clinic. Some clinics are loaning POCT devices to patients to explore self-monitoring or for use on holiday.
Counselling and patient education
Due to the many clinical and operational factors associated with warfarin therapy, providing the patient with comprehensive verbal and written education is essential. The NPSA recommends that information about anticoagulant medicine is given at various points of the patient journey: on initiation, on discharge from hospital, at the first anticoagulation clinic appointment and at regular intervals throughout treatment.5 At an initial consultation, it can be a challenge to get the right balance between ensuring patients’ questions and concerns are addressed and covering all relevant topics within an allotted time.
There is growing evidence that patients’ adherence is associated with their satisfaction with the information they have received about their medicines.7 Adherence is strongly linked to anticoagulation control; poor adherence will result in increased thrombotic and haemorrhagic events. Because the level of information that patients desire is likely to vary, information must be tailored appropriately to individuals. A variety of risk scores for thrombotic and haemorrhagic events as well as patient decision aids are available (eg, to consider the use of warfarin for AF).8 These tools may add value to consultations, resulting in more satisfactory dialogue and promoting a partnership approach and adherence. Key points to cover in patient education are listed in Panel 3.

The nationally recognised yellow oral anticoagulation therapy pack includes a warfarin information booklet. Patients are encouraged to read this at the start of therapy and to keep it as a resource to remind themselves of key aspects of their warfarin therapy. The booklet is available in a variety of languages (www.npsa.nhs.uk/health/alerts).
Practice points
Reading is only one way to undertake CPD and the regulator will expect to see various approaches in a pharmacist’s CPD portfolio.
- Make sure you understand how your local anticoagulation services operate as well as specific tools used (eg, counselling checklists) or policies followed. This minimises the risk of giving advice that conflicts with the clinic.
- Practise advising on the points in Panel 3.
- Ensure all patients on warfarin bring their warfarin record to the pharmacy.
Consider making this activity one of your nine CPD entries this year.
Patient-held records
Anticoagulation services are expected to provide patients with an up-to-date record of their latest INR test results, dosage information and next clinic appointment.7 Although most clinics provide patients with a yellow oral anticoagulation therapy record book for this purpose, NPSA guidance is not prescriptive on the format of the patient-held record.
Clinics using the yellow record books require patients to bring their book to each appointment so that it can be kept up to date. A small number of clinics use locally developed hand-written records which may contain more comprehensive information than the yellow book. With the development of computer-assisted dosing for warfarin therapy some clinics are opting not to use a hand-written record book but to provide patients with the specified information in the form of a computer-generated print out. Some clinics use computer-printed labels, which are stuck into the yellow book. Most computer systems have the ability not only to provide information on the latest INR result but also an INR and dosage history. Whatever method of documentation is used patients should be reminded to keep their records and to present them proactively to relevant healthcare professionals.
Ideally, patients should carry their records with them at all times but, if this is not feasible the oral anticoagulation pack contains a credit-card sized alert card that can be kept in a purse or wallet to serve as a method, in an emergency, of identifying the patient is on warfarin. Patients sometimes present their alert card rather than their book to the pharmacy and it should be explained that the pharmacist not only needs to know they are on warfarin but also needs to be able to review their management to ensure that the supply of medicines is safe.
Warfarin patients recently discharged from hospital frequently experience changes to their warfarin dose requirements. Reasons for this may include acute illness, change in diet or change in their medicines regimen. Patients are required to take their discharge summaries to their anticoagulation clinic appointments to ensure the clinic can make well informed decisions about warfarin therapy.
It should not be assumed that because a prescription has been written for warfarin that it is appropriate to dispense. Panel 4 describes how community pharmacists can review patient-held records.

New oral anticoagulant agents
Over the next few years a host of new oral anticoagulants will enter the UK market for use in stroke prevention in AF. Key characteristics of warfarin and some of the latest agents are outlined in Panel 5.
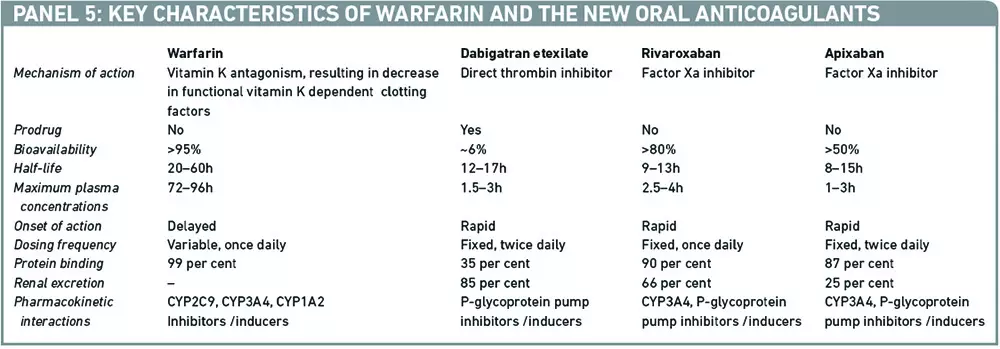
In general, in the selected trial populations data suggest that the newer agents are either non-inferior or demonstrate modest benefit over warfarin. In addition, they offer a number of advantages, such as predictable pharmacokinetics, no requirement for monitoring of blood clotting time, fewer drug interactions and standardised dosing. However these advantages are expected to come with a considerable price tag. Furthermore, as with all new drugs, there are uncertainties with regards to their long-term safety and effectiveness. Current concerns also exist around the lack of specific antidotes for these newer agents and the impact of withdrawing the regular monitoring component of oral anticoagulation on patients’ adherence.
Initial reviews of the data for the newer oral anticoagulants together with consideration of the cost implications and uncertainties indicate that warfarin continues to be the first choice oral anticoagulant for AF. However a managed entry approach that identifies and prioritises patient groups considered to benefit most from the newer agents is appropriate. Community pharmacists are well placed to respond to queries about the newer agents providing unbiased information and reinforcing local decisions on prescribing of the newer agents.
This article is intended to be read in conjunction with the P&MM article on p255.
Acknowledgements
Thanks to Mike Laffan, professor of haemostasis and thrombosis at Imperial College London, for his review and helpful comments.
References
- Williams KE, Gardiner C, Mackie IJ, Machin SJ, Cohen H. Preliminary data from the Medical Devices Agency (MDA) study of the CoaguCheck S in a patient self-testing environment. British Journal of Haematology 2003;121 (Suppl. 1):7.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Annals of Internal Medicine 2007;146:857–67.
- Monette J, Gurwitz JH, Rochon PA, Avorn J. Physician attitudes concerning warfarin for stroke prevention in atrial fibrillation: results of a survey of long term care practitioners. Journal of the American Geriatric Society 1997;45:1060–5.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Archives of Internal Medicine 1999;159:677–85.
- National Patient Safety Agency. Safety Alert 18: Actions that can make anticoagulant therapy safer. Available at www.npsa.nhs.uk (accessed 17 August 2011).
- Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C et al and the British Committee for Standards in Haematology. Guidelines on oral anticoagulation with warfarin — fourth edition. British Journal of Haematology, 2011;154:311–24.
- Horne R, Hankins M, Jenkins R. The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research. Quality in Health Care 2011;10:135–40.
- National Prescribing Centre. Atrial fibrillation patient decision aid. Available at www.npc.nhs.uk (accessed 24 August 2011).