Mclean/Shutterstock.com
Conditions caused by thrombosis (blood clots) will kill a staggering one in four people across the world[1]
, yet, unlike diseases such as cancer, thrombosis is not often taken seriously as a public health issue. However, the COVID-19 pandemic has pulled treatment for thrombosis into sharp focus.
Moderate-to-severe COVID-19 was soon recognised as a pro-inflammatory condition that presents with an increased incidence of venous thromboembolism (VTE), where thrombosis occurs in the deep veins, such as in the leg, groin or arm, and in the lungs (pulmonary embolism and in-situ pulmonary thrombosis)[2],[3]
. COVID-19 has also been recognised to increase the incidence of myocardial infarction and embolic stroke. VTE has been reported to occur in around 10−35% of COVID-19 patients in intensive care, while autopsies suggest it could affect up to 60% of patients[4]
.
In lieu of prospective data, centres across the UK have doubled the dose of anticoagulation used for VTE prophylaxis in patients with moderate to severe COVID-19, while other centres are using treatment doses of anticoagulation as VTE prophylaxis in severe COVID-19[5]
.
Alongside COVID-19 patients requiring anticoagulation, regular anticoagulation patients have also been affected. NHS England advised anticoagulant services to, where possible, switch patients on warfarin to take direct oral anticoagulants (DOACs) — it is unclear just how many patients have been switched — to reduce the need for regular blood tests and thereby reduce the risk of COVID-19 transmission[6]
. However, this approach comes with risks and this is not the only impact the pandemic has had on anticoagulation services.
As we quickly adapt to these changes, we must not forget that these medicines can be dangerous if managed improperly, particularly during transitions of care. After all, heparin and anticoagulants stand as the ‘H’ in ‘A-PINCH’, an acronym adopted by the World Health Organization (WHO) to recognise particularly high-risk medicines: Anti-infectives, Potassium, Insulins, Narcotics, Chemotherapeutic agents, and Heparins and anticoagulants (although this list is not exhaustive).
For the benefit of our patients — and if we hope to meet the WHO’s target of reducing severe avoidable medication-related harm by 50% across the world between 2017 and 2022[7]
— we should use this opportunity to take stock and think about how we can do things better in anticoagulation. Pharmacists should be at the heart of it.
Switching from warfarin to DOACs
Patients taking the anticoagulant warfarin require regular international normalised ratio (INR) monitoring, and these appointments could increase the risk of transmission of COVID-19. Therefore, in March 2020, the Royal Pharmaceutical Society published guidance on how to safely switch patients from warfarin to DOACs to limit face-to-face contact during the pandemic[8]
. However, owing to the scale and pace of this switch, preliminary data suggest that we should be alert to the risk of therapeutic duplication, with patients inadvertently receiving DOAC and warfarin together[9]
.
While DOACs require less regular monitoring, we should not assume that they are without risks: the Medicine and Healthcare products and Regulatory Agency’s drug safety update in June 2020 stated that bleeds associated with DOACs, which are often life-threatening or fatal, continue to occur[10]
. The update reminds us that DOACs should be used with caution in patients at increased risk of bleeding, such as older people and patients with low body weight or renal impairment. Also, creatinine clearance should be calculated using the Cockcroft-Gault equation to determine renal function for dosing of DOACs; estimated glomerular filtration rate may overestimate renal function and increase the risk of bleeds.
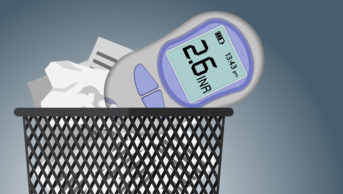
Raised INR risk with COVID-19
Some patients on warfarin may be awaiting a switch or may be unable to switch to an alternative and, where it is safe, intervals between INR monitoring should be increased for this group (but no less frequently than every 12 weeks[11]
).
However, there is emerging evidence that patients on warfarin who contract COVID-19 may have an increased incidence of high INRs in the inpatient and outpatient setting. One London centre has reported significantly increased INRs in this patient cohort[12]
, with associated fatal bleeds.
Switching patients from warfarin to a DOAC or a low-molecular-weight heparin (LMWH) may also require additional blood tests to ensure safety. Particularly in the outpatient setting, conducting switches in suspected or confirmed COVID-19 patients who are unwell presents an additional challenge; it may be necessary to seek specialist input to assess the right course of action.
The role of pharmacists in anticoagulation
These changes to regular practice present some challenges and this is where pharmacists come in.
Pharmacists improving adherence and optimising treatment
There is considerable evidence that adherence continues to be a major challenge for patients across disease areas associated with anticoagulation, such as (but not limited to) atrial fibrillation[13]
. Therefore, pharmacists and pharmacy technicians should play a role in counselling patients and checking their understanding. The patient must know what anticoagulation therapy is and why they must take it (that is, the indication and benefits of taking anticoagulation weighed against the risks of not taking it), and they must be given the opportunity to express their concerns and have these addressed. Adherence is also enhanced by involving patients in the decision-making process. Consideration should be given to their preferences, such as: once-daily or twice-daily dosing; whether the anticoagulant needs to be taken with food; whether it can be crushed; or whether it should be put into a blister pack or dosette box.
The pharmacy team can deliver annual reviews to patients. In addition to checking understanding and adherence, they can obtain an up-to-date review of thrombotic and bleeding risk, and either lead on or support decisions about whether the balance of risk favours continuation or cessation of anticoagulation.
Various models of pharmacist-led anticoagulation service have demonstrated the benefit of pharmacist involvement in optimising anticoagulation control, minimising adverse events, and providing a positive patient experience[14]
,[15]
.
Pharmacists improving safety
With a wide range of anticoagulant agents and dosing variations available, pharmacy should have a frontline role in reviewing prescribing and addressing errors such as wrong dose, therapeutic duplication, inappropriate interruption or inappropriate continuation of anticoagulation therapy.
In primary care, practice systems, such as EMIS, can be configured to alert clinicians regarding monitoring frequency and duplication of treatment on a patient’s list. In addition, practice pharmacists can set up bespoke alerts as reminders for dose reduction and stop dates to avoid patients falling through the net.
To enure prescribing, follow-up and monitoring are appropriate for all patients, it will be helpful to set up a list of patients receiving anticoagulants, to run regular searches on the practice list to identify further patients, and to review this list.
Pharmacists in primary and secondary care can ensure that patients on DOACs have their renal function determined appropriately using the Cockcroft-Gault equation. In the general practice setting, some prescribing systems, such as EMIS, have a built-in Cockcroft-Gault calculator. Where this is not available, online calculators such as MDCalc can be used.
Pharmacists supporting transfers of care
Pharmacy can also take the lead in supporting safety at transitions of care in several ways. Pharmacy can ensure the discharge summary from secondary care is comprehensive and includes indication, duration of treatment and stop date, dates of any dose changes and recent renal function. Work should be undertaken so that discharge letters are configured to include haemoglobin, serum creatinine, liver function test results and recent weight — useful information for anticoagulation services and primary care colleagues.
For patients who are newly started on anticoagulation in the secondary care setting, referral to community pharmacy for the new medicines service provides an opportunity to check patients’ understanding, answer queries and enhance adherence. Research suggests that patients followed up by community pharmacists after being discharged are less likely to be readmitted within 30 days[16]
.
During the COVID-19 pandemic, several centres issued extended thromboprophylaxis to patients discharged from hospital following a COVID-19 diagnosis with durations ranging from 14 days to 42 days: an unlicensed indication. As part of the transfer of care around medicines service in England, patients on extended thromboprophylaxis can be referred to their community pharmacy for support and advice, and to ensure that patients do not receive anticoagulation for this indication for longer than intended[17]
.
The discharge medicines service will be piloted during 2020, allowing hospitals to digitally refer recently discharged patients to community pharmacies in England for advice on new and updated prescriptions, and this will go live in 2021[18]
.
Pharmacy should lead the creation of COVID−anticoagulation pathways to support prescribing as patients move across settings. It is also important for pharmacy to communicate to patients who have been switched from warfarin to another anticoagulant that they should take any unused warfarin to their community pharmacy for disposal to avoid inadvertent use of two anticoagulants. Pharmacy can also support the arrangement of continued repeat prescriptions for patients switched from warfarin to LMWH, particularly in settings where there are shared care arrangements or LMWH prescribing is red-listed in primary care.
Pharmacy should take the lead in developing and implementing guidance, safe systems and pathways to support management of anticoagulation in and across different settings: for example, including stop dates on prescribing instructions, and outlining clear follow-up requirements and the process of managing patients who do not attend follow-up so that they are not discharged without involvement and support from primary and secondary care.
As medicines experts, there is an opportunity for pharmacy professionals across secondary care and primary care, including community pharmacy and primary care network professionals, to work together to provide integrated care in anticoagulation.
Frances Akor, consultant pharmacist in anticoagulation, Imperial College Healthcare NHS Trust
Acknowledgements: Amanda Da Costa, GP prescribing support and care home pharmacist, Ilford
References
[1] Loranzo R, Naghavi M, Foreman K et al. Lancet 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
[2] Costela-Ruiz V, Illescas-Montes R, Puerta-Puerta J et al. Cytokine Growth Factor Rev 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001
[3] Levi M, Thachil J, Iba T et al. Lancet Haematology 2020;7(6):E438–E440. doi: 10.1016/S2352-3026(20)30145-9
[4] Manolis AS, Manolis TA & Manolis AA. J Cardiovasc Pharmacol Ther 2020. doi: 10.1177/1074248420958973
[5] British Thoracic Society. 2020. Available at: https://www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/bts-guidance-on-venous-thromboembolic-disease-in-patients-with-covid-19/ (accessed September 2020)
[6] Malson G. Pharm J 2020;305(7940):90–93. doi: 10.1211/PJ.2020.20208223
[7] World Health Organization. 2012. Available at: https://apps.who.int/iris/bitstream/handle/10665/80058/A65_REC1-en.pdf?sequence=1&isAllowed=y (accessed September 2020)
[8] Williams H. 2020. Available at: https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Coronavirus/FINAL%20Guidance%20on%20safe%20switching%20of%20warfarin%20to%20DOAC%20COVID-19%20Mar%202020.pdf?ver=2020-03-26-180945-627 (accessed September 2020)
[9] Medicines and Healthcare products Regulatory Agency. 13 October 2020. Available at: https://www.gov.uk/government/news/warfarin-and-other-blood-thinners-reminder-on-safe-use-during-covid-19-pandemic (accessed October 2020)
[10] Medicines and Healthcare products Regulatory Agency. 2020. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/896274/June-2020-DSU-PDF.pdf (accessed September 2020)
[11] Specialist Pharmacy Service. 2020. Available at: https://www.sps.nhs.uk/articles/management-of-patients-currently-on-warfarin-during-covid-19/ (accessed September 2020)
[12] Speed V, Patel RK, Byrne R et al. Thromb Res 2020;192:73–74. doi: 10.1016/j.thromres.2020.05.024
[13] Salmasi S, Loewen PS, Tandun R et al. BMJ Open 2020;10(4):e034778. doi: 10.1136/bmjopen-2019-034778
[14] Dowling T, Patel A, Oakley K et al. Pharm J 2016. doi: 10.1211/CP.2016.20201474
[15] Ingram SJ, Kirkdale CL, Williams S et al. BMC Health Services Research 2018. doi: 10.1186/s12913-018-2901-8
[16] Wilcock M, Sibley A, Blackwell R et al. Int J Pharm Pract 2020. doi: 10.1111/ijpp.12603
[17] Wickware C. Pharm J 2020. doi: 10.1211/PJ.2019.20206557
[18] Wickware C. Pharm J 2020. doi: 10.1211/PJ.2020.20208305