
Life in View / Science Photo Library
Executive summary
The handling of controlled drugs (CDs) within the hospital setting requires skill and attention to detail. The legal framework, designed to reduce criminality whilst enabling access to healthcare for patients, is complex, and does not take account of technological developments.
There are many specific processes involved with these medicines, which take up considerable staff resource and require regular training and updating in policy and procedures.
The new technologies include automated dispensing systems in both pharmacies, wards, and departments, and these, linked to digital systems, have made progress with the handling of CDs. The new technologies should be recognised within the legal framework and guidance and be promoted as a more efficient and secure means of ordering, storing, and supplying CDs.
Recommendations
- That the new technologies, including automated dispensing systems for CDs, be recognised in guidance controlling the handling of CDs;
- That the ordering, storing, and supplying of CDs in hospitals should be through automated dispensing systems;
- That the need for a prescriber’s wet signature on electronic patient discharge and outpatient prescriptions should be reviewed;
- That the paper-based CD Record book should be replaced with electronic registers;
- That the messenger transporting CDs from the pharmacy to a clinical area should use a digitalised system for recording their movements;
- That when the messenger is a registered healthcare professional, they could put the CDs into the automated dispensing system and verify the quantity in stock with the authorised user in the clinical area. When the messenger is not a healthcare professional, the CDs should be delivered to the ward and the placing of the CDs into the automated dispensing system is done by an authorised healthcare professional;
- That there be a nationally recognised, consistent approach to automation and digitalisation in the handling of CDs, accompanied by CD policy and CD Standard Operating Procedures (SOPs);
- That there be an inclusive training programme for the handling of CDs using automation and digitalisation for all affected healthcare staff.
Introduction and background
Controlled drugs (CDs) are essential to modern clinical care. They must be stored securely, and their use requires specific ordering processes and chronological detailed records in a designated bound register. The legal framework for the management and use of CDs in the hospital setting is a minimum standard, and the Home Office enforces the legislation for robust arrangements for the management and use of CDs via the police to minimise patient harm, misuse, and criminality. The Care Quality Commission (CQC) and General Pharmaceutical Committee (GPhC) also have roles to play in regulating the framework and the practitioners who are acting in the environment in which CDs are handled.
Challenges with management of CDs in a hospital, including the pharmacy, wards, and departments, include time-intensive mandatory record keeping processes and physical reconciliation of stock, often further compounded by inadequate storage facilities and capacity.
UK hospital pharmacies have sought to increase the use of robotics, and while there are limited published data on quantitative outcomes, the consensus is that they have provided benefits through a reduction in dispensing errors and improvements in dispensing efficiency/stock management, increased availability of staff time through an impact on staff time required for dispensing and availability of technician time at ward level for clinical work, and reductions in dispensary space[1]. For hospitals planning to build new pharmacies, or remodel existing facilities, a robot is suggested to form the centrepiece, underpinning the medicines management process[2].
Automation is available in a variety of models from different vendors, but the USA Institute for Medication Practices (ISMP) believes that the safe use of the technology can only be achieved through the adoption of standard practices and processes. The ISMP has produced a set of 9 guidelines to support organisations in decision making and strategic planning for the safe use of automated dispensing cabinets (ADCs). Following completion of the guidelines, the ISMP commented that they are now “far more comfortable with the ability of ADCs to support the safety of the medication distribution system while making required drugs readily accessible in a variety of patient care areas. However, this is not to say that there are not continued improvements to be made or potential pitfalls associated with the use of these devices, particularly if workflow expectations are not well designed or defined. Clearly, if remaining risks to safety are not properly managed, patients can be placed in jeopardy”[3].
Robotics systems have only been used for CDs in a limited and trial basis in a very small number of UK hospitals due to conceptual difficulties in complying with legislative storage and record keeping requirements. Therefore, most hospitals leave CDs to be managed through traditional lock and key, cupboards, and paper ledgers for documentation[4].
The recently published Health Building Note (HBN) 14-02 provides best practice guidance on storage facilities for medicines, including CDs in clinical areas, clarifying the legislative and regulatory requirements around medicines storage and it provides new guidance on automated drug storage cupboards[5].
The adoption of this updated guidance, and the widespread adoption by the NHS of standards to ensure the safe handling of CDs in terms of ordering, supply, storage, administration, and disposal when using technology, will improve consistency in handling and reduce waste in processes. A framework under which SOPs and guidelines could operate, to ensure all statutory requirements governing CDs are followed and met is warranted.
This document reviews the existing legislation and guidance on CDs and considers robotic storage and supply of these medicines. The report proposes to assist in the debate and offer a way forward as legislation continues to develop.
The current legal framework for handling CDs in hospitals
The term ‘controlled drug’ is defined by the Misuse of Drugs Act 1971 as ‘any substance or product for the time being specified in Part I, II or III of Schedule 2 of the Misuse of Drugs Act 1971’.
The legislation applicable to CDs and pharmacy include:
- The Misuse of Drugs Act 1971
- The Misuse of Drugs Regulations 2001
- The Misuse of Drugs (Safe Custody) Regulations 1973
- The Health Act 2006
- Controlled Drugs (Supervision of Management and Use) Regulations 2013.
The enforcement body for CDs offences is the Home Office, via the police. The Misuse of Drugs (Safe Custody) Regulations 1973 detail the storage and safe custody requirements for CDs.
The Health Act 2006 introduced the concept of an ‘Accountable Officer’ and requires healthcare organisations, and those providing services to healthcare organisations, to have standard operating procedures in place for using and managing CDs. CD Accountable Officers (CDAOs) are responsible for supervising and managing the use of CDs in their organisation. Designated bodies must appoint a CDAO who will quality assure processes for managing CDs in their organization, in line with Regulation 8 of the 2013 Regulations.
For registered pharmacies, the Responsible Pharmacist Regulations 2008 also require that a range of pharmacy procedures be established including procedures for CDs. However, legislation no longer details which specific CD SOPs should be in place[6]. RPS Professional Guidance on the Safe and Secure Handling of Medicines requires that up-to-date organisational policies and procedures are in place covering the management of CDs such as security, ordering and receipt, record keeping, prescribing and clinical monitoring, administration, supply, denaturing and disposal, use and storage of patients’ own CDs, transport and investigation and reporting of concerns[7].
The CQC regulates against all “Regulations of the Health and Social Care Act 2008 (Regulated Activities)”. The aim of Regulation 12 of the Act is to prevent people from receiving unsafe care and treatment and prevent avoidable harm or risk of harm. As part of the duty of co-operation set out in these regulations, the Trust contributes to and participates in a Local Intelligence Network for Controlled Drugs, or ‘CD LIN’. The Trust is required to share an ‘occurrence report’ that provides detail of concerns that the Trust has regarding the management or use of CDs by any relevant individuals (or that it has no concerns), as set out in the NHS England Single Operating Model.
Healthcare professionals and service providers who are required by the 2001 Regulations to maintain a CD register must have an authorised person present to witness the destruction of stock CDs in Schedule 2 in line with Regulation 27 of the 2001 Regulations. The appointment of authorised witnesses for the destruction of CDs is a role for the CDAO.
Principles relating to handling CDs
Principles of administration of CDs
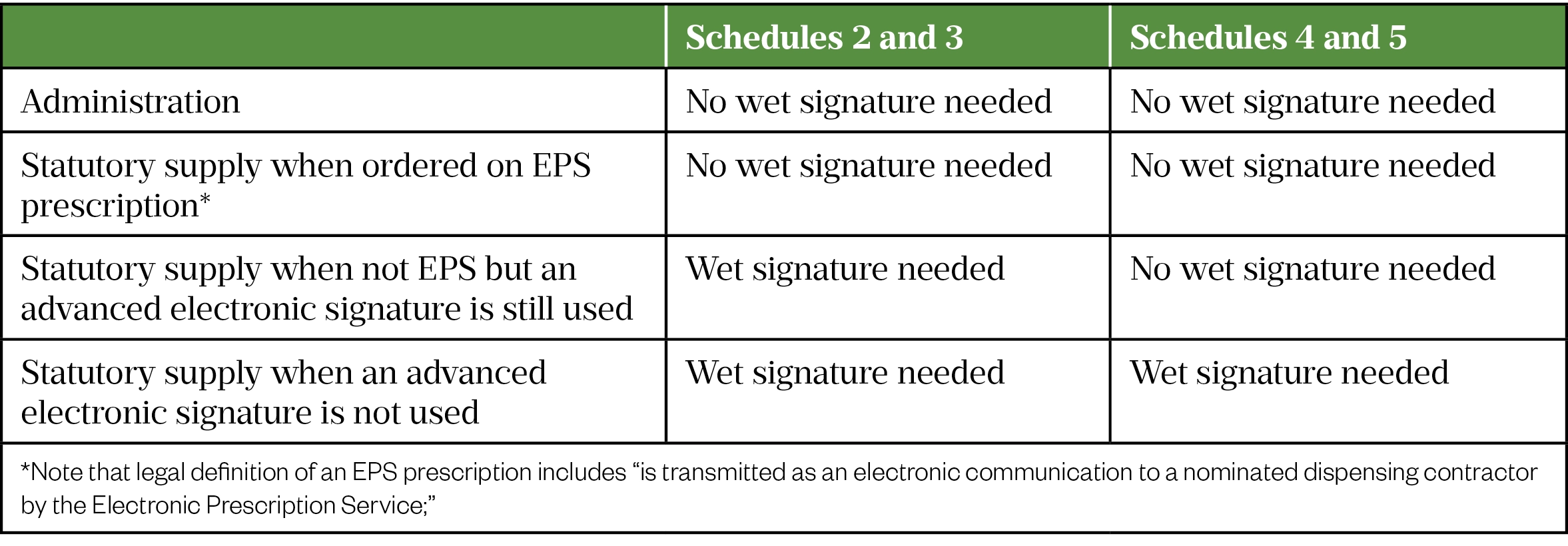
Schedule 2, 3 or 4 CDs can be administered to a patient by a doctor, dentist, pharmacist independent prescriber or nurse independent prescriber acting in their own right, or a supplementary prescriber acting in accordance with a clinical management plan, or a person acting in accordance with the directions of a prescriber entitled to prescribe CDs, (there is no requirement for a wet signature here).
Principles of prescription requirements for the statutory supply of schedule 2 and 3 CDs
The prescription requirements relating to statutory supply of schedule 2 and 3 CDs for discharge or outpatient supply for example, and these are as follows:
- Signature – The prescription needs to be signed by the prescriber with their usual signature. Advanced electronic signatures can be accepted for Schedule 2 and 3 CDs where the Electronic Prescribing Service (EPS) is used.
- Date – The prescription needs to include the date on which it was signed. CD prescriptions are valid for 28 days after the appropriate date on the prescription. The appropriate date is either the signature date or any other date indicated on the prescription (by the prescriber) as a date before which the drugs should not be supplied – whichever is later.
- Prescriber’s address – The address of the prescriber must be included on the prescription and must be within the UK. In hospitals, the address is the hospital trust.
- Name of the CD – It is good practice to write the name in full as it appears in the manufacturer’s summary of product characteristics.
- Dose – The dose does not need to be in both words and figures; however, it must be clearly defined.
- Form – The formulation must be stated; the abbreviations ‘tabs’ and ‘caps’ are acceptable.
- Strength – The strength only needs to be written on the prescription if the medicine is available in more than one strength
- Total quantity – The total quantity must be written in both words and figures.
- Quantity prescribed – The Department of Health and the Scottish Government have issued strong recommendations that the maximum quantity of Schedule 2, 3 or 4 CDs prescribed should not
- exceed 30 days. If supply does exceed 30 days, there must be a documented clinical reason for this.
- Name of patient.
- Address of patient.
- Dental prescriptions – Where the CD prescription is written by a dentist, the words ‘for dental treatment only’ must be present.
- Instalment direction – Where the prescription is intended to be supplied in instalments a valid instalment direction is required
Benefits of prescribing CDs through the Electronic Prescription Service
Community pharmacies can dispense CDs via the Electronic Prescription Service (EPS) except instalment dispensing of CDs. The benefits of prescribing CDs through the EPS include:
- Fewer patients with both paper and electronic prescriptions, making it easier for them as all their prescriptions can be sent electronically to their nominated pharmacy;
- Sending more prescriptions electronically will reduce the administrative burden on both GP practice and pharmacy staff;
- Prescriptions will be sent securely and electronically, and so cannot be lost or misplaced;
- Being able to see everything that has been prescribed helps pharmacists make the right decisions to dispense the right drugs safely and effectively for patients;
- Patients who had chosen not to use EPS because they still had paper prescriptions for CDs can now benefit;
- Patient safety is increased as errors are less likely.
Principles for CD ordering
Legislation requires that a requisition “in writing” must be obtained by the supplier – i.e., the pharmacy, before delivery of any stock Schedule 2 or Schedule 3 CDs to recipients such as a senior nurse in charge of a ward or a department. The requisition must be in writing, signed by the recipient, and specify the total quantity of the CD to be supplied and the requisition should be marked in such a manner to show that it has been complied with.
There are several situations where it is isn’t specific as to whether “written” is referring to electronically written or written by hand on paper and written electronically has been widely interpreted. Also, the term “signed” is ambiguous and has not been changed in the legislation since 2001, and this has been interpreted to be signed electronically.
Legislation requires that a requisition in writing must be obtained by the supplier (i.e., the pharmacy) before delivery of any Schedule 2 or 3 CDs to recipients such as hospitals or senior registered nurse in charge of wards or departments. We interpret this to mean that an electronic requisition meets the legislation.
Where stock is collected by a messenger on behalf of a purchaser (i.e., a ward senior nurse), a written authorisation must be provided to the supplying pharmacist that empowers the messenger to receive the medicines on behalf of the purchaser. We interpret this to mean that an electronic written authorisation meets the legislation. The supplying pharmacist needs to be reasonably satisfied that the authorisation is genuine and must retain it for two years.
When a requisition for a Schedule 2 or 3 CD is received by a person responsible for the dispensing and supply of medicines at a hospital, they must mark and retain the original requisition for two years. It is our view that an electronic request fulfils this requirement.
On 30 November 2015, amendments to the Misuse of Drugs Regulations 2001, made the use of an approved mandatory requisition form (FP10CDF which is downloaded from the NHSBSA website) to request stock of Schedule 2 and 3 CDs in the community[6,8]. When FP10CDF forms are used, the requisitioner normally has to use a unique identifying code to identify themselves (not a requirement of the regulations but discussed on the form). This means that a medical prescriber requires an individual prescriber code for each different NHS practice they work in. The NHSE/I CDAOs issue these unique identifiers for practitioners practising privately. The NHSE/I CDAOs want people who don’t have a code directed to be directed to them.
Following the introduction of the form, the Home Office was made aware that activities within the hospital sector which would normally be governed by provisions under regulation 14(6), and which were not expected to come within the scope of the new requirement, were being captured because of changes in the NHS structures e.g., Trust mergers. This was an unintended consequence of the changes to NHS structures and healthcare delivery since 2011 rather than a result of a regulatory change.
The requirement to use a mandatory form was not expected to extend to the hospital environment where supplies of CDs were undertaken by the hospital trust pharmacy. Additional guidance was therefore issued to further explain how the provisions governing the use of the new mandatory form may be interpreted. This guidance does not impact on the need for a Home Office licence[8].
Where a requisition is received by a hospital pharmacy within the same legal entity, the approved form is not required, and the existing arrangements may be continued.
Where the request is from a different legal entity the approved form must be used. However, the Home Office has advised that the person in charge of a hospital can issue a yearly bulk requisition on the approved mandatory form to the separate legal entity that supplies its wards / departments to then draw on throughout the year using CD requisition books with duplicate pages. The Home Office advises that supplies from one registered pharmacy to another registered pharmacy should only be made after receiving a written requisition on an approved requisition form.
Principles for safe custody and storage of CDs
The Misuse of Drugs (Safe Custody Regulations) 1973 refer to the physical security of certain Schedule 2 or 3 CDs. It requires that pharmacies keep relevant CDs in a ‘locked safe, cabinet or room which is constructed as to prevent unauthorised access to the drugs’. This applies to registered pharmacies and care homes. The requirement does not extend to other settings, but these standards are expected as a minimum requirement. This is notable as other settings have more discretion about how they store CDs.
HBN 14-02 sets out recommendations and standards for CD cupboards and their security/access plus recommendations where range and volumes of CDs is high[5].
For automation of CDs, HBN 14-02 recommends local discussion about automated electronic medicines storage and issuing systems[5]. The HBN states that Regulator guidance is currently limited as to security levels and approval. Discussion with local police and home office inspectors is recommended before implementation together with risk assessment.
When CDs requiring safe custody are not kept in the CD cabinet, safe or room (e.g., during the dispensing process), they must be under the ‘direct personal supervision’ of a pharmacist. A key log could be used to keep an audit trail of who has had access to the keys, including overnight storage in the pharmacy, the transfer of the keys from one pharmacist at the end of a shift to another pharmacist, etc.
Principles for department, ward, or unit CD stock lists
Each department or ward will agree a CD stock list with the Pharmacy. The list will be determined with the appropriate practitioners working in that clinical area. The contents of this list will reflect current patterns of use of CDs in the ward or department and only CDs on the department, ward / unit stock list should be requisitioned / ordered routinely. The list should be modified if practice changes and will be reviewed annually.
Principles for access to CDs
Following requisition from pharmacy, once CDs reach the ward, they become the responsibility of the authorised regulated healthcare professional in Charge on duty and must be immediately stored securely in the CD cupboard. All CDs must be stored in a locked receptacle which can be opened only by a person who can legally be in possession, such as a pharmacist or the registered nurse/midwife in charge or a person working under their authority. Note that any person may be in possession, where that possession is for the purpose of conveying the drugs to another person that may have those drugs in their possession and this is why a hospital porter could legally possess CDs as long as that were accepted local policy.
Keys for the CD cupboard should be held by the nurse or midwife in charge of the ward or department. The person in charge is responsible for controlling access to the CD keys and access to all CD cupboards on that Ward or Department for that shift.
Staff authorised to order CDs
The registered nurse/midwife in charge of a ward or department, operating theatre or theatre suite is responsible for the requisitioning of CDs for the use in that area. Even if the ward or department is managed by someone other than a nurse or midwife, under the present regulations the most senior nurse or midwife present is responsible for CDs.
CD orders for stock supplies are usually written in the Ward CD Order Book (ref. 90-500) and the person ordering the CD stock must ensure that the order in the Ward CD Order Book is completed in ink and that the carbon copy has printed correctly. In some hospitals alternative arrangements are in place, including “top up” or separate stationery. The Ward CD Order Book should be kept in the CD cupboard when not in use or in a securely locked place.
Principles for record keeping for CDs
A CD register must be used to record details of any Schedule 2 CDs received or supplied by a pharmacy. (Pharmacists are also required to keep records of Sativex). Legislation requires that the class, strength, and form be specified at the head of each page of the CD register.
The register is required to be a bound book register although electronic CD register are now recognised. It is also a requirement that different classes are kept in a separate part of the register and that, within each class, a separate page is used for different strengths and formulations of each drug.
Electronic CD registers
These are permitted as an alternative to having a bound-book CD register. Legislation requires that computerised entries must be:
- Attributable
- Capable of being audited
- Compliant with best practice (Goldberg, 2004). (The Medicines, Ethics and Practice Guide 2019 does not define “best practice”).
An electronic CD register must also be accessible from the premises and capable of being printed. Registers may only be kept in computerised form if safeguards are incorporated into the software to ensure all the following:
- The author of each entry is identifiable
- Entries cannot be altered later
- A log of all data entered is kept and can be recalled for audit purposes.
Access control systems should be in place to minimise the risk of unauthorised or unnecessary access to the data. Adequate backups must be made of computerised registers. Arrangements should be made so that inspectors can examine computerised registers during a visit with minimum disruption to dispensing.
Running balances and stock checks
A running balance should be maintained as a matter of good practice and is a recommendation from the Shipman Inquiry. It is intended that once electronic registers are in common use this will become a legal requirement.
The aim of a running balance is to ensure that irregularities or discrepancies are identified as quickly as possible. There should be SOPs in place for checking stock levels against the running balance and dealing with discrepancies. For most organisations, the frequency of stock checks should be at least once a week, but these may be more or less frequent based on risk, volume of CDs dispensing, frequency of past irregularities or incidents, or if there are several different pharmacists in charge over short periods. Liquid balances should be checked visually with periodic volume checks and checks to confirm the balance on completion of a bottle.
Stock checks should be recorded, signed, and dated by the healthcare professional carrying out the check and if possible, two people should carry out stock checks. It is also appropriate to visually check the running balance each time a CD is dispensed (i.e., where the calculated balance in the register visually matches the quantity, you can see. If it does not match, the discrepancy should be investigated in more detail).
In most wards and departments in secondary care, a daily CD stock check is conducted and both ward stock and patients’ own CDs are checked. The stock check will usually be conducted by one registered nurse supported by another competent healthcare professional to witness the stock check.
A CD stock inspection should be conducted in wards and departments approximately every 6 months by pharmacy staff together with a registered nurse / midwife. The outcome of the inspection should be made available to the CDAO.
Principles for the transportation of CDs
Where stock is collected by a messenger on behalf of a “purchaser”, a written authorisation must be provided to the supplying pharmacist that empowers the messenger to receive the medicines on behalf of the “purchaser”. The supplying pharmacist needs to be reasonably satisfied that the authorisation is genuine and must retain it for two years.The stock must be entered into a CD Record book on delivery by an authorised nurse. The stock balance must be checked to ensure it agrees with the actual stock present in the CD cupboard.
Principles for the destruction and disposal of CDs
Pharmacies are required to denature CDs prior to disposal. Usually, this process requires an appropriate license, but pharmacies can register an exemption without needing to obtain a license. In England and Wales, an exemption is issued by the Environment Agency and is known as the ‘T28 exemption’. This allows pharmacies to sort and dispose of CDs and to comply with the 2001 Regulations by denaturing them prior to disposal. The Home Office has advised that all CDs in Schedules 2, 3 and 4 (part 1) should be denatured and, therefore, rendered irretrievable before disposal.
In the case of obsolete stock of schedule 2 CDs, the denaturing of CDs needs to be witnessed by an authorised person. Various individuals and classes of person (e.g., police constables, GPhC inspectors, regional pharmacists) are authorised to witness the destruction of schedule 2 CDs. A CDAO has the power to authorise other persons to witness the destruction of CDs. Persons authorised by the CDAO are usually senior members of staff who are not involved in the day-to-day management or use of CDs. Note also that holders of Home Office licenses for possession of CDs may have witnesses authorised by the Home Offices named on their license.
Safe custody applies to patient-returned out of date and obsolete CDs until they can be destroyed. To minimize the risk of supplying these to patients the stock should be segregated and clearly marked.
The destruction of patient-returned CDs whether they require denaturing or not, does not require witnessing by an authorised person, however it is preferable for denaturing to be witnessed by another member of staff familiar with CDs (preferably a registered health care professional). A record of the destruction of patient returned CDs should not be made in the CD register, but records of patient returned schedule 2 CDs and their subsequent destruction should be maintained in a separate record for this purpose.
The destruction of expired or unwanted schedule 2 CD stock must be witnessed by an authorised person, and an entry should be made in the CD register.
Current problems in handling CDs in hospitals
Current problems with handling CDs in hospitals are numerous and are in large part brought about through time-intensive, mandatory record keeping processes and physical reconciliation of stock, which involve people at every stage in the process and storage facilities are frequently inadequate. To manage this situation, as a minimum all hospitals must have their own robust overarching CD policy in addition to risk-assessed, task specific SOPs.
Information on the outcomes of problems with CD handling in hospitals is sparse and anecdotal. The number of incidents reported to NHS England CDAOs, for primary care (who report incidents directly to NHSE) was, for the first time, published by the CQC for the period 2018/19. There were 2899 unaccounted losses of CDs on the reporting tool (Care Quality Commission, 2019 ) and nearly half of these reports (1387) involved CDs being lost, stolen, or missing[9,10]. The CQC reported that because of the staggered roll-out of the reporting tool across NHS England regions, the data was subject to variation in the detail reported and the interpretation of risk by organisations, but there was an average of 8 daily unaccounted for losses recorded across England[9].
The training of pharmacy professionals and nurses is key to the safe and secure management of CDs. This includes initial education about the legal framework and guidance for CD handling but also includes regular updating of the contents of SOPs which must be aligned to the law; the practice of the registrants needs to be kept up to date through regular updating and therefore staff training is relevant at every stage in the handling of CDs.
CD storage
For pharmacy and clinical areas there should be processes for environmental monitoring of CD storage, review of security (especially keys), segregation of high strength stock, handling patient’s own CDs and expired CDs. HBN 14-02 recommends that all cupboards should be made of metal, and that they comply with BS 2881 Security Level 2, or the “silver level” outlined in Secure Standard 314 and that the requirement for alarm systems and other security measures are dependent upon local risk assessment. To access control to CDs the HBN recommends that the lock to the room or cupboard complies with BS 3621 and that electronic keys and appropriate electronic access cards are preferred to ensure suitable audit trails of storage access can be maintained. These current regulations around steel thickness and safe rooms will need to be reviewed if CDs are to be stored in automated locked cabinets with secure locking bins controlled by fingerprint entry. The current type, location and size of storage space can be problematic and require investment to correct. Also, it is variable which drugs are stored in CD cupboards in clinical areas in accordance with local risk assessment.
CD ordering and supply
There should be SOPs in place to ensure requests for CDs are legal, that CDs requested are approved for stocking and order volumes are appropriate. The Responsible Pharmacist regulations state that procedures also should be in place to ensure the arrangements to secure the handling of medicinal products including CDs.
The authorisation process for individual staff members allowed to order CDs by location, the identification of authorised staff and the handling of keys are problematic because they
are time consuming to operationalise and this leads to inconsistency. The CDs which are approved for stocking in a particular location requires constant review as does the restocking process.
There are different methods of ordering CDs for use in clinical areas including the CD Record book, a “top up” system, or an automated route involving the email system or an order direct to the Pharmacy Information System. The frequency of requests for replenishment of stock can be ad hoc or pre-planned and the stock quantity may not be standardised for ordering.
There can be “stock CDs” and “non -stock” CDs and the authorisation process to change the stock lists for an individual location are problematic to operationalise.
Stock may not be available when needed for a patient and may be delayed in dispatch from the pharmacy resulting in missed doses of essential agents.
CD prescribing
SOPs should be in place to ensure the legality of prescriptions and safe prescribing practices with reduced opportunity for dependency, overdose, and diversion.
The training of prescribers and pharmacy teams in the intricacies of CD prescribing is problematic in the hospital setting with frequent problems occurring with the form of the agent prescribed and the quantities not meeting the legal framework. Also, the traceability of paper prescriptions is a problem. The use of electronic prescribing in primary care has addressed some of these concerns.
CD recording
There should be SOPs in place to ensure a full audit trail of each movement of a CD. The problems include errors in the records and a complex means of error correction. The need for a witness to sign the record book for stock movements including administration, and destruction of part used doses is problematic.
The method of recording can now involve an electronic register in addition to a bound paper book. The location of the book should be in the CD cupboard, but books can go missing or be damaged with ripped or missing pages. Using an automated system also improves control of infection through not requiring paper records.
Audit of the CD record keeping is important, but of a variable nature, and may involve daily or weekly review, and utilise independent pharmacy assessment on a quarterly or 6 monthly – basis.
CD transport
The movement of CDs to and from the pharmacy department must be fully auditable and risk assessments should be undertaken to determine people authorised to transport CDs. The problem areas include the variety of methods used for recording the identification of the deliverer from a signature in the CD Record book to a signature on a top up sheet designed specifically for an individual location. Upon delivery of stock from pharmacy to a clinical area, the stock must be recorded in the Record book, but finding the authorised staff member for doing that on a busy ward can be problematic. Using an automated electronic system will therefore free up valuable staff time, both the messenger’s time and the recipient’s time.
CD destruction and disposal
All facilities disposing of CDs must have a T28 waste exemption certificate issued by the Environment Agency. Care must be taken to ensure that they are not open to abuse, and part used products should have their contents withdrawn and denatured.
All destruction needs to be witnessed. The staff who are authorised to destroy and dispose of CDs need to be known and they need to be aware of the method of denaturing and destruction. The record keeping of the destruction and witness arrangements need to be in place and robust.
Audit provision
There should be SOPs to monitor all operational processes including review of prescribing trends and high-volume prescribing.
Diversion of CDs is a continued source of concern to the CQC who wrote in June 2018 that ‘Nationally there is a general naivety about diversion issues’ advising that health and care staff should consider regular monitoring and auditing arrangements for CDs in the lower schedules to identify and take swift action on diversion and governance and reporting of concerns should be included as part of commissioning and contracting arrangements[11]. Diversion of schedule 4 and 5 CDs is very common and often goes undetected.
Software systems, such as ADIoS are available to facilitate completion of CD audits.
Raising concerns and sharing learning experiences
There should be guidance related to specific current and topical issues available to aid professional decision making. Any concerns about misuse of CDs must be reported and CDAOs should be assured that they or their representative will be sighted on these. A duty of candour applies to staff who should be encouraged to raise any concerns they may have relating to the management of CDs. A just culture should be fostered, and staff should be encouraged to speak up.
The optimal way to order, store and manage CDs
The optimal way to handle CDs is to store them securely, with limited access to authorised individuals only, using a closed end to end system for automated ordering and supply. There needs to be a digital record keeping system which records who has accessed the stock, the stock that has been taken and what has happened to the stock. These need to be consistent and standard processes occurring in a timely, non-paper-based manner. In addition, a standard staff training package focused on the different professionals involved is required.
The greater use of digital systems and automated drug cabinets will improve the audit trail, provide controlled access, and create consistency in processes. Automated dispensing systems can enhance the efficiency of CD workflow and dispensing between wards and pharmacy.
Automation and digitalisation have developed and have started to address some of the issues which accompany the handling of CDs. Now is the time for the legal framework and guidance such as Medicines, Ethics and Practice Guide to recognise the progress made with these systems and be updated accordingly to keep pace with technological developments.
The opportunities of managing CDs in a digital age
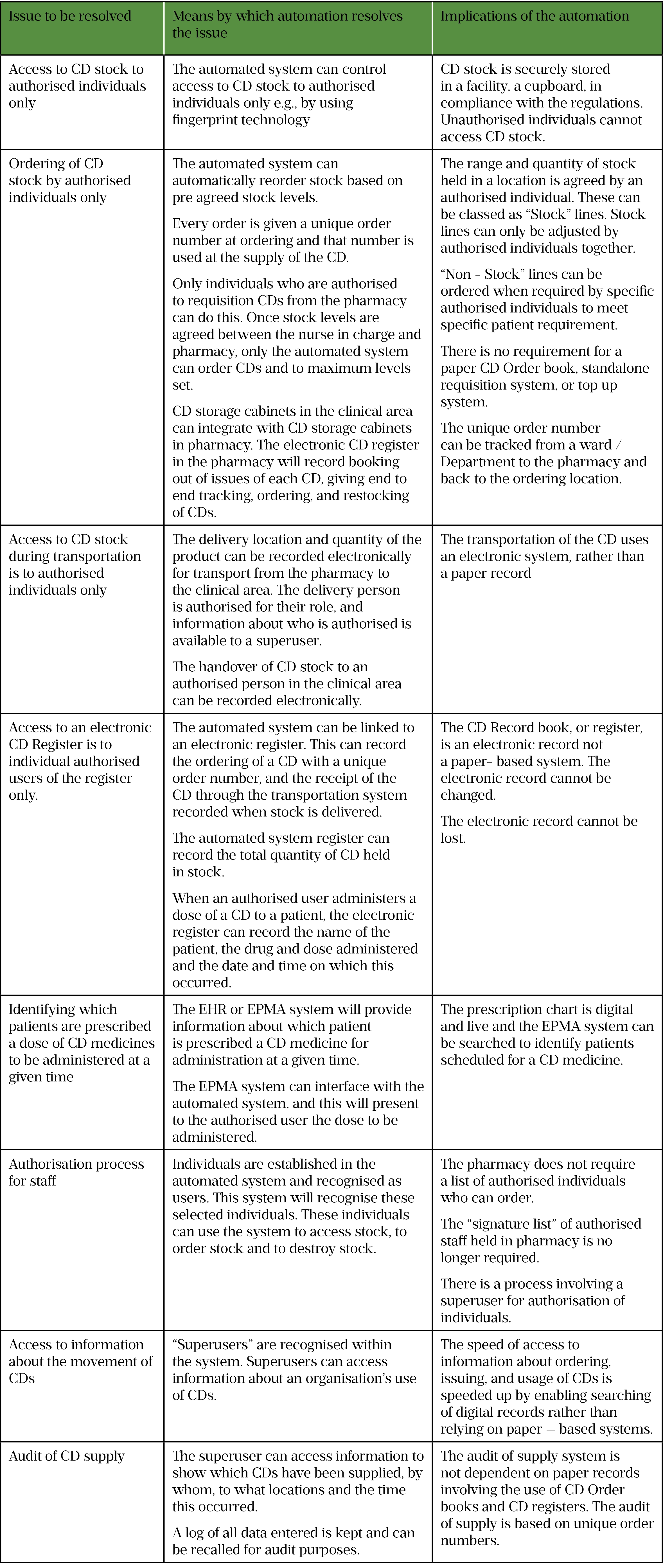
What does automation and digitalisation offer to the handling of CDs in hospitals now? Overall, an automated CD storage device with a digital recording system will provide an audit trail showing who has handled what. The overall summary of what the automated system can do is shown below and in Table 1.
- An automated cabinet can secure stock from access to all but authorised individuals
- The range and quantity of stock held in a location is agreed by an authorised individual and these can be classed as “Stock” lines. “Non – Stock” lines can be ordered by specific authorised individuals.
- The location and quantity of the product can be recorded, both in automated storage devices and during transportation, and related information is available to authorised people.
- Each order for each CD supplied can have a unique number associated with it.
- Automated CD storage cabinets in clinical areas can integrate with CD storage cabinets in pharmacy. The order for a CD from a clinical area can be digitalised and actioned within the CD automated storage device in the pharmacy – thus creating a unique order without a paper trail.
- The digital system can integrate with automated systems for clinical end to end tracking, ordering, and restocking and will automate the ordering of stock – using predetermined stock levels and accounting for stock movements.
- An electronic CD register in the pharmacy can record booking out issues of each CD, giving end to end tracking, ordering, and restocking of CDs.
- A digital system can record the messenger for each delivery of CD from pharmacy to a clinical area. On delivery, an authorised person can sign for receipt of the CD on the digital system.
- A digital recording system can provide an audit trail to show who has issued what CD
- Batch number of stocked items supplied can be recorded in the automated system
- The automated system can integrate with the ePMA or EHR and will determine which drugs are needed for which patients and which wards.
- A pharmacy to wholesaler order can be generated by the automated system based on stock levels approved by the authorised user, using a unique order number at ordering and delivery of goods.
- Report on any discrepancies with stock
- Time savings of not searching for keys or stock which leads to better nursing and pharmacy workflows and improved patient experience and care.
Optimum automated system using digitalisation for use in the hospital setting?
There are currently a variety of automated systems available in the hospital setting. Some hospitals use automated systems in the pharmacy, some use the automated systems on the wards, and a few use an integrated system where the wards and the pharmacy automated systems are fully integrated.
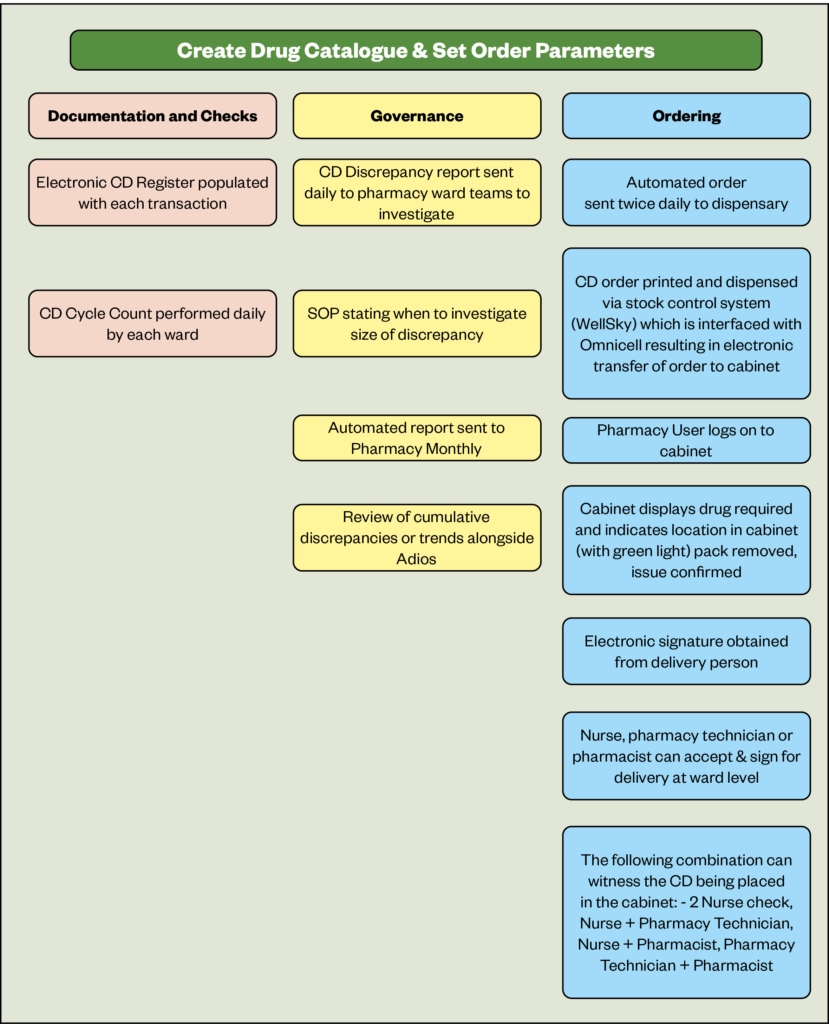
An optimal system involving Omnicell cabinet use in Chesterfield Royal Hospital is shown diagrammatically below. This system uses digitalisation and automates the ordering and supply of CDs, the delivery, and the restocking of the cabinet in the clinical area. These principles are applicable to other automated systems.
The supply of CDs to clinical areas is governed by historical usage patterns which create a stock list and an ordering history within the automated cabinet. Twice daily, an order from the clinical area robot is despatched to the robot in pharmacy. Some hospitals use digital ordering directly between the clinical area and the pharmacy, some use a CD Order book, or an email system to order the CDs required. The preferred option is the one using the smallest number of steps. A digital order, based on usage patterns, is transmitted directly between the automated cabinets on the ward and in the pharmacy, and this is interfaced with the stock control system in the pharmacy.
The staff in the pharmacy receive the order for the CD and supply the products from the automated cabinet. An electronic delivery note is prepared, which could use fingerprint technology in its production, and the stock is despatched to the clinical area via a messenger.
The messenger could be a pharmacy assistant, a pharmacy technician or a pharmacist or a nurse. More information about the processes observed in Chesterfield Royal Hospital using a variety of staff, is in section 10.4. Once in the clinical area, the stock is entered into the automated cabinet by the messenger or a receiving nurse. Control on access to the stock is then handed over to the nurses in the ward or department.
What next for automation and digitalisation in the handling of CDs in hospitals?
Developing and establishing systems and processes for organisations
The Standards for handling CDs should be updated to enable digitalisation and automated cabinets to be recognised. Those with a role to play here include the Home Office to set the legal framework, the GPhC and the CQC.
Organisations should agree governance arrangements for CDs with clear lines of responsibility and accountability, and that should include the use of automation and digitalisation. The CDAO, who will quality assure the processes for managing CDs and will liaise with the local NHSE lead CDAO, must approve the organisation’s CD policy and procedures from the perspective of automation and digitalisation.
Record keeping for organisations
The legal framework requires a separate CD register to be kept for each storage location and these along with requisitions for CDs must be kept for 2 years. These records can be kept on paper or preferably electronically on the organisation’s pharmacy information system. The content of electronic records would be available through digital searching using tools supplied with the record keeping system.
Risk assessment for organisations
Organisations should carry out a risk assessment to determine whether CDs in schedule 3,4 and 5 should be handled as those in schedule 2. We would strongly recommend that this includes weak opioids such as codeine, and morphine sulfate 10mg/5ml solution, and benzodiazepines.
The classification of CDs should be noted in SOPs which will determine how these products are handled. The CDs can be stored in robots and handled digitally as schedule 2 CDs.
Processes for reporting incidents relating to CDs
Local and national systems for reporting CD incidents should be used in organisations but a local process should be used to record incidents related to automation and digitalisation. The CDAO must be kept informed when implementing automated and digital systems and the CDAO should be especially vigilant during the change over from paper, lock, and key based systems.
Prescribing CDs
Prescribing CDs in hospitals is currently facilitated with electronic prescribing, but a wet signature is required for all schedule 2 and 3 CDs to be taken away from the hospital. This situation needs to be changed to facilitate electronic prescribing for outpatients and at patient discharge. Advanced electronic signature can be used for schedule 4 and 5 CDs.
Sufficient CDs should be prescribed to meet the patient’s clinical needs for no more than 30 days and the patient’s GP must be informed of all prescribing decisions and record this in the patient care record so that the GP has access to this information.
Obtaining and supplying CDs
Supply of CDs using an automated system simplifies adherence to legal requirements both in the pharmacy and on wards and in departments. There are some inconsistencies in the application of automated systems at present which presents an opportunity for a move to a consistent process.
In the pharmacy department
In pharmacy, the ordering of CDs from wholesalers and manufacturers, and receipt of CDs should follow the principles of good procurement. Local procedures should ensure that there is a robust audit trail and that the opportunities for diversion are minimised. An automated system can alert procurement when an order is required, or automatically place an order with the supplier or wholesaler. Where orders are placed on the pharmacy system, this can interface with and display the pending order numbers and supplier on the system. Supplier details and delivery information can be automatically recorded in the e-register on receipt.
In wards and departments
The stock levels and preparations of CDs held in wards and departments are required to match what is routinely used in that clinical area. Using automation, real time usage data can quickly assess trends in usage and recommend stock levels for optimum balance of availability of drugs through appropriate stock holding. This reduces workload for ward staff and pharmacy staff.
In most NHS hospitals currently, orders are written on suitable stationery (e.g., a controlled drug requisition book with duplicate pages, or a locally determined standard requisition tool) and must be signed by an authorised signatory. A copy of the signature of each authorised signatory should be available in the pharmacy department for validation. However, using automation, the tool for ordering is an electronic request, based on the stock level of an individual CD in an individual location. The benefit of the automated approach is that there is no requirement for a signature to be referenced against copy in pharmacy to confirm authority of signatory. Where electronic systems are in use, there should be a reliable means of validating the identity of individuals who requisition CDs. Further the automated system is the only system that can order CDs for the ward, and so the automated interface will be used as validation for ordering and each order for a CD will be given a unique order number.
Those using automated CD systems currently do not use them consistently. In respect of ordering, some order direct to the pharmacy information system in the pharmacy, others order direct to pharmacy via a printer / email in a formatted CD Order book, whilst some print an order on the ward on paper and dispatch it to pharmacy and finally some still use a traditional CD Order book. There is an opportunity for everyone to use a consistent process and that which involves an order from a ward / department cabinet being made directly to pharmacy via the pharmacy information system seems like the process involving the least number of steps and avoids using paper.
Transporting CDs from pharmacy to wards / departments
The person who accepts the CDs for transit should sign for receipt. This may be on the duplicate requisition (if space permits) or may be in a separate book kept for this purpose. Electronic tools are now available, for use in a dispensary, that can record electronically the identity of the person accepting receipt of the CDs either by photograph or signature (on electronic signature pad). This results in no ambiguity of staff through poor handwriting and therefore decreased risk of fraudulent activity.
When the messenger transporting the CD from pharmacy to the clinical area in a tamper evident bag / locked container is ready to hand over the stock to an authorised individual there are some risks. The messenger may not be able to find the authorised individual and the supply may not be placed into the automated cabinet in a timely, correct manner.
The handover process may require some amendment. For example, the messenger could transport the CDs to the clinical area and enter the stock directly into the automated facility. The handover process could then be via a digital record of the new stock levels in the automated system.
Administering CDs
When administering CDs, it is important for relevant standards set by the professional regulator to be followed and where necessary that any safety concerns are checked with the prescriber. Providing information and advice to people having CDs administered and recording the administration are essential elements of care.
The record of administration must include the name of the person having the dose, the date and time and the name formulation and strength of the agent, along with the dose administered and the name and signature of the person administering the dose and that of any witness to the administration. It is important to record wastage and for disposal of that to be witnessed, e.g., 5mg of morphine administered from 10mg ampoule. This is a common way to divert CDs, especially in theatres
The electronic register can record this information.
When an EPMA system is used, this can interface to the automated system and both identify that a dose of a CD is scheduled and can supply that individual dose to the authorised user.
Handling CDs
Records of handling CDs are required to provide an audit trail for the supply, administration and disposal of CDs and the movement from one location to another.
The organisation’s SOPs require there to be a clear audit trail for the movement and use of all CDs. Automation and digitalisation provide an integrated pathway covering the steps from ordering to administration, which are recorded, including dispensing, delivery, and receipt and all of these are accounted to a user.
Access to CDs is restricted to appropriate, designated and legally authorised personnel, who can access the CDs with electronic access e.g., fingerprint.
The stock balance of all CDs entered in the CD record book should be checked and reconciled with the amounts in the cupboard with sufficient frequency to ensure that discrepancies can be identified in a timely way. Using automation, the stock balance is confirmed at every transaction. Alert notices print when a discrepancy is found and can automatically be emailed to designated personnel immediately for timely resolution.
Monitoring the use of CDs
This activity is undertaken to prevent discrepancies and diversion. When discrepancies are detected, a number of checks should occur. These include that all requisitions have been entered into the correct page of the register, that all CDs administered have been entered into the CD register, that items have not been accidentally put into the wrong place in the cupboard and the arithmetic should be checked to ensure that stock balances have been calculated correctly.
In an automated stock management situation, all entries of ordering, dispensing, delivery, receipt of issue and arithmetic are automated. This will decrease the risk of errors, discrepancies and save time and ambiguity due to automated record keeping.
Automated ordering means that the risk of selecting the wrong product to put in an automated location is further reduced as it is pre-set from the order. The system could be set to scan stock being put into the system to confirm the correct product is being placed in the correct location.
Overall, an automated CD storage device with a digital recording system will provide an audit trail showing who has handled what and when.
What are the benefits of using an automated system for handling CDs?
Automated systems reduce the risk of fraudulent activity by securing access to CDs to authorised individuals. They also support simplification of arrangements around associated tasks such as ordering, distribution, stock management, record keeping and audit. In a Covid age, the automation of CDs brings with it an opportunity to support infection control by avoiding using paper.
There have been a small number of benefits described and published following the implementation ofautomated systems handling CDs.
Leeds Teaching Hospitals NHS Trust – personal contact
Leeds Teaching Hospitals was an early adopter of a pharmacy-based robot, an ADC, and reported a reduction in time to dispense CDs by 50%, a streamlined dispensing process reducing from 9 steps to 6, and a cost saving through staffing skill mix changes.
Belfast Health and Social Care Trust
A project based in Belfast Health and Social Care Trust (BHSCT) has demonstrated the safety and effectiveness of an ADC in managing CDs (Hughes, Slee, & Farrar, 2002). Prior to installation of the cabinet there had been a 20% increase in CD activity from 2013 to 2018, and this along with the Carter target of a 15-day stock holding for medicines, affected working conditions, and the high volume of work led to errors which in turn increased staff anxieties when assigned to CD tasks. Automation reduced the time taken to supply CDs by 63% and the error rate was reduced from 24 per 1000 to 9 per 1000 dispensed items. New errors were operational and dealt with through a training program; the previously recorded documentation errors were eliminated. Future developments were to focus on linking ward-based ADCs with the pharmacy ADC to further enhance the efficiency of controlled drug workflow between ward and pharmacy. BHSCT plans to install controlled drug ADCs in all its hospital pharmacies.
University Hospital Coventry and Warwickshire – personal contact
In 2018/19 the Trust procured and installed 56 automated medicines cabinets, including automated medicines cabinets for the pharmacy CD room and pharmacy emergency room. In terms of dispensing times for CDs, electronic registers replaced paper registers in the pharmacy, and the time taken for a single CD transaction was reduced by an average of 1 minute 48 seconds. Over a week this was a time saving of over 15 hours of pharmacy technician time.
Chesterfield Royal Hospital – personal contact
Amongst many examples of advanced clinical practice, we observed at Chesterfield Royal Hospital was the full integration in the professional roles between the ward-based pharmacy technicians and the nursing team around the handling of CDs.
This 550-bedded hospital has automated cabinets on 5 wards and a further 3 cabinets storing CDs in the Accident and Emergency department.
In response to an order automatically generated from pre-agreed levels from an automated cabinet in the form of an email, the pharmacy technician enters the request onto pharmacy dispensing system to manage the stock level and create a CD product package label (containing the order number and the planned location) and then picks the order direct from the automated cabinet in the pharmacy. A digital system is used to create the delivery note and the messenger is despatched with the CD to the clinical area. When the messenger is a pharmacy technician, on arrival on the ward the stock is placed within the automated cabinet and the digital register recorded the new stock balance. When the messenger is an assistant, the stock is handed over to a nurse to enter the automated cabinet.
A pharmacy technician was observed working on the ward to supply CDs from the automated cabinet, in response to an order on the EPMA. Here the pharmacy technician would supply the CD to the nurse to administer to the patient and act as a “witness” or hand the CD over to the nurse to administer and a second nurse would be the witness, and the digital register would account for their actions accordingly.
The audit of CDs in the clinical area involved a daily stock count. With the implementation of automation, the audit activity was reported to take less than 50% of the time previously taken.
Conclusions
The overall aim of the legal framework around CDs is to reduce criminality, whilst the regulations are in place to enable healthcare with CDs for patients.
The handling of CDs in hospitals is currently fraught with problems. There is a considerable burden of administrative processes involving paper-based systems, and diversion issues are a source of concern. Staff training is a considerable workload for organisations as is maintenance of CD policies and SOPs.
The legal framework is permissive in the handling of CDs in secondary care, and frequently it is the interpretation of the law that leads to bureaucratic processes when it comes to handling CDs. An example of this problem relates to the writing of discharge prescriptions. It is a commonly held belief that the whole of the CD prescription requires to be written by hand, whereas in fact it is only a wet signature on an electronically generated prescription that is required by law. The prescription may be signed by another prescriber and still be legally valid, (although the address needs to be applicable to the signatory for the prescription) and the register entry should record the detail of the actual prescriber – the signatory – rather than the named prescriber.
In many organisations it is the discharge prescriptions involving CDs that adversely impacts on timely patient discharge by creating a significant bureaucratic workload for pharmacy and medical staff. Implementing the wet signature of a prescriber on an electronically produced discharge prescription would reduce these time constraints, better still would be an electronic signature.
New technologies are available, involving automated dispensing cabinets and digitalisation, both in the pharmacy and clinical areas and go a long way to improving the complexity of handling CDs. There is no change required in the legal framework for automated and digital systems to be implemented, although guidance for chief pharmacists around the interpretation of the law would be useful.
Some hospitals have invested in these new technologies. There is now a need for a more widespread adoption of change and investment in technologically advanced equipment. Every hospital should be reviewing its handling of CDs and implementing automated and digital systems, and this is to reduce the workload associated with supplying CDs and to increase the governance on the handling of these products in the hospitals setting.
In line with this is a requirement for a national CD policy for hospital handling of CDs, national SOPs and a national training programme for all staff involved with handling these medicines. Such guidance would cater for exceptional circumstances, such as the rare but allowable situation in which in an emergency a verbal order for the administration of a schedule 2 or 3 CD which is followed up retrospectively in writing.
The guidance governing the handling of CDs in hospitals requires updating to bring it into line with technology available. The Royal Pharmaceutical Society’s Medicines, Ethics and Practice Guide needs to reflect the current and developing practice in the hospital setting. The legal framework requiring a wet signature on prescription stationery for CDs to be taken out of the hospital by a
patient needs to be changed and an option for this could be to implement electronic prescription transfer technology to the hospital setting as is used in primary care.
The technology is here to support the handling of CDs in our hospitals; this article seeks to summarise the current legal situation and encourage chief pharmacists and regional pharmacists of the urgency to implement automated and digital system that will reduce the workload, increase the ability to comply with the law and overall reduce criminality.
Acknowledgements
The authors would like to thank Omnicell for sponsoring their work.
And the following individuals for their comments and observations which have provided invaluable inwriting this report:
- Alison Brailey, Chief Pharmacist, Chesterfield Royal Hospital;
- Tracy Draper, Principal Pharmacy Technician, Chesterfield Royal Hospital;
- Laura Rodgers, Ward Services and Systems Lead Pharmacy Technician, Chesterfield Royal Hospital.
- 1Implementing an automated dispensing system. Pharmaceutical Journal. 2002. doi:10.1211/pj.2021.1.90210
- 2Goldberg LA. Automation in hospital pharmacies. Hospital Pharmacy Europe. 2004.https://hospitalpharmacyeurope.com/news/editors-pick/automation-in-hospital-pharmacies/ (accessed Apr 2022).
- 3Guidelines for the Safe Use of Automated Dispensing Cabinets. Institute for Safe Medication Practices. 2019.https://www.ismp.org/resources/guidelines-safe-use-automated-dispensing-cabinets (accessed Apr 2022).
- 4O’Kane A, Francis W, Duffy C, et al. Implementing an automated dispensing system for the safe management of controlled drugs. Hospital Pharmacy Europe. 2021.https://hospitalpharmacyeurope.com/news/reviews-research/implementing-an-automated-dispensing-system-for-the-safe-management-of-controlled-drugs/ (accessed Apr 2022).
- 5(HBN 14-02) Medicines storage in clinical areas. NHS England. 2021.https://www.england.nhs.uk/publication/hbn-14-02-medicines-storage-in-clinical-areas/ (accessed Apr 2022).
- 6Medicines, Ethics and Practice. 44th ed. London: : Royal Pharmaceutical Society 2021. https://www.rpharms.com/publications/the-mep
- 7Safe and secure handling of medicines. Royal Pharmaceutical Society. 2018.https://www.rpharms.com/recognition/setting-professional-standards/safe-and-secure-handling-of-medicines (accessed Apr 2022).
- 8Safer management of controlled drugs (CD). NHS Business Services Authority. 2017.https://www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/prescribing-and-dispensing/safer-management (accessed Apr 2022).
- 9The safer management of controlled drugs. Care Quality Commission. 2019.https://www.cqc.org.uk/sites/default/files/20190708_controlleddrugs2018_report.pdf (accessed Apr 2022).
- 10Open to abuse: gaps highlighted in the controlled drug system. The Pharmaceutical Journal. 2019. doi:10.1211/pj.2019.20207251
- 11The safer management of controlled drugs. Care Quality Commission . 2018.https://www.cqc.org.uk/sites/default/files/20180718_controlleddrugs2017_report.pdf (accessed Apr 2022).



