It was billed as a treatment worthy of a Nobel prize. In 2013, two studies purportedly showing the effectiveness of oral antibiotics in treating back pain were published in the European Spine Journal1,2. Given that 10–17% of the population in England report back pain, which is often chronic and difficult to treat, the findings were accompanied by enthusiastic coverage in the mainstream media3.
At the time, The Guardian reported that “up to 40% of patients with chronic back pain could be cured with a course of antibiotics rather than surgery, in a medical breakthrough”4.
One medic quoted in the story — Peter Hamlyn, a consultant spinal surgeon in London — was full of praise. “More work needs to be done but make no mistake, this is a turning point, a point where we will have to rewrite the textbooks.”
He even went as far to suggest that the NHS could potentially save £250m by using antibiotics rather than surgery4.
However, 12 years later, there is no Nobel Prize, nor are antibiotics for chronic back pain part of routine NHS care. Instead, the 2013 studies have led to a dispute within the scientific community. Critics argue that the bacteria identified in the studies were most likely spread via contamination and antibiotic treatment is best avoided, given the global threat of antimicrobial resistance. Meanwhile, several of the initial researchers, together with Hamlyn, went on to help create the company Persica Pharmaceuticals to focus on developing a patented “injectable, thermosensitive” antibiotic formula for back pain5.
Modic changes
In the first 2013 study, a group of UK and Danish researchers reported that they had found anaerobic bacteria — “predominantly Propionibacterium acnes” (now known as Cutibacterium acnes) — in 43% of 61 patients having lumbar discectomy for disc herniation. Of those patients, 80% developed changes in the vertebrae adjacent to the previous disc herniation1. These are called ‘Modic changes’ (MCs), representing changes in the marrow of the end plates of vertebrae, as seen on MRI scans. MCs are graded according to MRI appearances (see Box).
Box: What do Modic changes mean?
- MC-1 shows signs of the body reacting to stress or injury, such as repair processes or inflammation;
- MC-2 is marked by the buildup of fat in the area;
- MC-3 involves the hardening of bone tissue, which can push out the normal bone marrow and make the bone look denser6.
The clinical significance of these changes is debated.
The second 2013 paper reported on a randomised controlled trial (RCT) of 162 patients with back pain for at least six months and whose MRI scans revealed MC-1 changes2. Participants were randomised to 100 days of antibiotic treatment (Bioclavid [amoxycillin + clavulanic acid]; Sandoz) or placebo. One year later, the researchers reported that the antibiotic group showed statistically significant reductions in scores for back and leg pain, disability scores and ‘general improvement’. No conflict of interest was declared on the papers.
According to Hamlyn, these findings were exciting because it was “a simple generic antibiotic” that was able to target the bacteria.
Commenting during a Guardian webchat at the time, he said: “One of the beauties of their therapy is that it does not need injections — it is a simple, though long, course of tablets.”7
In partnership with study author Hanne Albert, Hamlyn went on to set up a paid-for training programme to certificate clinicians in something they termed ‘Modic antibiotic spinal therapy’ (MAST). However, while it is still possible to find a few private clinics still in the UK offering antibiotics for MCs, the certification programme has since withered8.
This research also led to the formation of UK company Persica in 2013. Steve Ruston, its chief executive, told The Pharmaceutical Journal that the idea behind Persica was to develop a new treatment for chronic back pain that would be acceptable to patients, regulatory agencies and the pharmaceutical industry. Albert and Claus Manniche, authors of the 2013 studies, are founding members of the company, while Hamlyn is a board member5,9.
Antibiotics and resistance
Aware of the problems linked to long-term, high-dose oral antibiotics — such as side effects, patient compliance challenges and the potential for increasing drug resistance — Ruston said the team behind Persica decided to try something different: developing a “patented, targeted intradiscal antibiotic injection” to be delivered directly to the site of infection.
A spokesperson from Persica told The Pharmaceutical Journal it is “fully committed to responsible antibiotic stewardship”.
“Our PP353 intradiscal injection reduces antibiotic exposure by 99.9% (0.3g versus 150–300g oral course) while achieving 5,000 x higher concentration at the infection site,” they added. The treatment involves two injections given during the first week.
In March 2025, Persica issued a press release of a phase Ib trial that showed that, after one year, patients with chronic back pain and Modic type 1 changes experienced significant pain relief with the treatment. Their pain dropped by 3.4 points (about 50%) on an 11-point scale, compared to a 1.4-point drop (about 30%) in the placebo group.
The treated group started seeing meaningful pain relief from 3 months and kept improving at 12 months, while the placebo group did not see much change until around 6 months. The full paper is currently under peer review at a journal.
Lloyd Czaplewski, chief scientific officer at Persica, describes their hypothesis, which is that C. acnes releases propionic acid during metabolism and triggers a local inflammatory response. This inflammation affects nearby nerves, including the sinusoidal nerve in the vertebra, contributing to pain. The bacteria also degrade bone — the Modic changes — which worsen the pain. Their theory is that using antibiotics will transform the management of back pain, which would improve the lives of millions of people.
He also told The Pharmaceutical Journal that there were minimal systemic side effects because so little of the drug is absorbed into the blood — an advantage over oral antibiotic studies where some people reported weeks of diarrhoea.

Charlotte Gurr
Scientific disputes
But the idea that bacteria are even present is disputed. During Persica’s development of its injection, several papers were published that cast doubt on the wisdom of pursuing an antibiotic treatment for back pain10,11. Persica, however, continued with its treatment.
Peter Fritzell, associate professor of the Centre for Spine Surgery in Stockholm, is one of the researchers who has taken issue with the concept of whether antibiotics can help.
After the 2013 publication, he went on to research whether bacteria are really a common cause of chronic back pain. Along with his colleagues, they compared tissue from spinal discs that had degenerative changes to tissue from discs with no degenerative changes (patients having surgery for scoliosis) — and found no difference between them. They published their findings in 201910.
“We found more or less exactly the same bacterial findings [in both groups of patients],” he says “most probably a consequence of contamination during the surgical procedures”.
With his colleagues from the University of Gothenburg, Sweden, they compared samples from young patients undergoing spinal surgery for scoliosis (curvature of the spine and no disc degeneration) with people having surgery for lumbar disc herniation (with degenerative changes).
They also took samples from the skin and surgical incision sites. On tissue analysis, they found bacterial growth — but in the same proportion of patients from each group. In other words, the presence of bacteria was not associated with degenerative back pain. Further, the positive samples were associated with the same bacteria in higher quantities on skin samples — C. acnes. They suggested this pointed to contamination as the source.
Gothenburg researcher Tomas Bergström explains that C. acnes is a common skin bacteria. “It can cause serious infections, like skin diseases in severely ill or immunocompromised patients — but it is not proven to cause distal infection like in the disc. This agent is a known contaminant,” he tells The Pharmaceutical Journal.
Olle Hagg, another of the Gothenburg researchers, adds that even if the skin is well cleaned before taking a biopsy, it does not work 100% to remove bacteria. “The alternative conclusion is that it’s completely normal to have some bacteria in the discs — from the teenage period onwards. But that seems improbable and we also know that sterilisation does not get rid of all the bacteria in the skin and especially not in the sebaceous glands, specifically not Cutibacteriae,” he says.
Other studies have used proteomics to identify bacteria and inflammation associated with Modic changes12. Unlike the traditional use of petri dishes and ‘broth’ to culture microbiomes, or the more modern genetic analysis to identify them, this identifies proteins associated with different bacteria. Proteomics cannot identify specific bacteria in samples, only the proteins associated with its activity.
However, Bergström is far from convinced that this can represent proof. “Theoretically you can use proteomics,” he explains. “But it’s not a primary diagnostic method — you can’t rely on them”.
“Many of these researchers really believe in their message, they are not doing this as ‘cheaters’, but they are trying to find support for their hypothesis,” he adds.
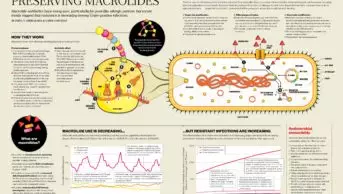
Persica’s Czaplewski is dismissive of this argument, arguing that the wrong methods have been used to detect very low levels of bacteria that lead to the inflammation. He maintains that high-quality microbiological studies consistently identify bacteria in 45% of disc samples when appropriate detection methods are used.
Persica points to histological studies “showing bacteria embedded deep within disc tissue in micro-colonies definitively rules out contamination”13,14. Animal models show that bacterial injection leads to Modic changes, Czaplewski adds.
Moreover, the presence of bacteria will not be used diagnostically. Instead, the need for antibiotics will be determined by a clinical history — ruling out other reasons for back pain, such as fractures or cancer — and the use of MRI to detect MCs, he says.
Back pain controversy
Yet, the meaning of Modic spinal changes as seen on MRI is controversial. A 2022 systematic review found that the quality of evidence in studies examining the link between Modic changes and back pain was poor in six studies and moderate in seven. “No conclusions can be drawn about an association between MCs and [lower back pain]… Currently, clinicians should not look for the presence or absence of MC to guide their management of patients with LBP,” the authors wrote15. Others have found similar16.
Hagg suggests that the term ‘Modic 1 changes’ makes the condition seem mysterious and special. Before any antibiotic treatment is used, bacteria should be found, he argues.
“In our view, there is no scientific evidence of Cutibacteriae infection of the intervertebral disc or of antibiotic treatment effect of ‘Modic 1 changes’ substantiating the development of disc injection or any other kind of antibiotic treatment,” he says.
Even in the original trials that found benefits of antibiotic treatment for Modic 1 changes, Hagg questions the meaning of them for patients. Not only is the evidence for infection in the discs very low, but it is questionable whether treatment with antibiotics help patients, in reality.
However, results from a 2019 Norwegian study, which replicated the original research by Albert et al., showed that patients taking amoxicillin had only slightly better scores on a back pain disability score questionnaire after one year compared with those taking a placebo — around a 1.6 point difference, which was barely statistically significant11. This result applied to patients with both Modic type 1 and type 2 changes.
In patients with type 1 Modic changes, the difference was bigger — about 2.3 points in favour of amoxicillin and, as critics were keen to point out, statistically significant. However, the measurement was on a 24-point scale, with the authors concluding that this was unlikely to be clinically significant: “Our results do not support the use of antibiotic treatment for chronic low back pain and Modic changes.”
Czaplewski told The Pharmaceutical Journal that despite there being statistically significant changes in those with Modic 1 changes, the Norwegian authors sought to “minimise the results”. He alleges they have done this deliberately because the authors “don’t like the idea of antibiotics to be used in this patient group”.
He adds it is driven by the concern of antibiotic resistance, particularly regarding the use of “such large doses of antibiotic over such a long period”. But the Persica product is different — it’s much more targeted than in the other trials, he clarifies.
Antibiotic resistance
For Fritzell and colleagues, the problem of potential overuse of antibiotics is enormous. They fear the result may be an increased and widespread use of antibiotics.
True infections in bone such as osteomyelitis are rare, he says, and this is quite different. There, you expect a complete resolution with antibiotics — which is quite different from this situation.
Bergström argues that the effectiveness of Persica’s antibiotic is still an open question until they have completed the phase III trial. “I think it is wrong by Persica to present data from a phase IIb trial as scientific evidence of effect, and to push for registration at this stage,” he says.
It is understandable that patients with long-lasting back pain want to try all possible ways to get rid of their problems but, in this case, the scientific evidence is not robust enough so far.
“We have to think about our children and their children,” he says. “We’re concerned about overuse of antibiotics and antibiotic resistance. We need antibiotics for the future that will work for even common infections in children and adults.”
The theory is interesting and research-worthy — and the stakes are high. But, given the sharp disagreements and big claims, independent systematic reviews and RCTs free of conflicts of interest are likely to be the only way to determine whether this could herald a revolution — or whether it is another story of overinflated claims and false hope.
- 1.Albert HB, Lambert P, Rollason J, et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J. 2013;22(4):690-696. doi:10.1007/s00586-013-2674-z
- 2.Albert HB, Sorensen JS, Christensen BS, Manniche C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J. 2013;22(4):697-707. doi:10.1007/s00586-013-2675-y
- 3.Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6):e010364. doi:10.1136/bmjopen-2015-010364
- 4.Sample I. Antibiotics could cure 40% of chronic back pain patients. The Guardian. May 2013. Accessed November 2025. https://www.theguardian.com/society/2013/may/07/antibiotics-cure-back-pain-patients
- 5.Our team. Persica Pharmaceuticals . Accessed November 2025. https://persicapharmaceuticals.com/our-team/
- 6.Udby PM, Modic M, Elmose S, et al. The Clinical Significance of the Modic Changes Grading Score. Global Spine Journal. 2022;14(3):796-803. doi:10.1177/21925682221123012
- 7.Back pain discovery: live webchat. The Guardian. May 2013. Accessed November 2025. https://www.theguardian.com/society/2013/may/07/antibiotics-cure-back-pain-webchat
- 8.What is MAST? Spine Surgery London. Accessed November 2025. http://www.spinesurgerylondon.com/patient-information/modic-antibiotic-spinal-treatment/what-is-mast
- 9.Medical Advisory Group. Persica Pharmaceuticals. Accessed November 2025. https://persicapharmaceuticals.com/medical-advisory-group/
- 10.Fritzell P, Welinder-Olsson C, Jönsson B, et al. Correction to: Bacteria: back pain, leg pain and Modic sign—a surgical multicenter comparative study. Eur Spine J. 2019;29(1):196-197. doi:10.1007/s00586-019-06199-4
- 11.Bråten LCH, Rolfsen MP, Espeland A, et al. Efficacy of antibiotic treatment in patients with chronic low back pain and Modic changes (the AIM study): double blind, randomised, placebo controlled, multicentre trial. BMJ. Published online October 16, 2019:l5654. doi:10.1136/bmj.l5654
- 12.Rajasekaran S, Tangavel C, Aiyer SN, et al. ISSLS PRIZE IN CLINICAL SCIENCE 2017: Is infection the possible initiator of disc disease? An insight from proteomic analysis. Eur Spine J. 2017;26(5):1384-1400. doi:10.1007/s00586-017-4972-3
- 13.Capoor MN, Ruzicka F, Schmitz JE, et al. Propionibacterium acnes biofilm is present in intervertebral discs of patients undergoing microdiscectomy. Brüggemann H, ed. PLoS ONE. 2017;12(4):e0174518. doi:10.1371/journal.pone.0174518
- 14.Ohrt‐Nissen S, Fritz BG, Walbom J, et al. Bacterial biofilms: a possible mechanism for chronic infection in patients with lumbar disc herniation – a prospective proof‐of‐concept study using fluorescence in situ hybridization. APMIS. 2018;126(5):440-447. doi:10.1111/apm.12841
- 15.Hopayian K, Raslan E, Soliman S. The association of modic changes and chronic low back pain: A systematic review. Journal of Orthopaedics. 2023;35:99-106. doi:10.1016/j.jor.2022.11.003
- 16.Herlin C, Kjaer P, Espeland A, et al. Modic changes—Their associations with low back pain and activity limitation: A systematic literature review and meta-analysis. Grasso G, ed. PLoS ONE. 2018;13(8):e0200677. doi:10.1371/journal.pone.0200677