Susumu Nishinaga / Science Photo Library
Most of us take breathing for granted. Air enters and exits the labyrinthine airways of our lungs instinctively. But for individuals suffering from asthma, breathing can be an epic battle. During a severe asthma attack, the thin tubes that shuttle oxygen and carbon dioxide constrict and fill with mucus, making inhalation nearly impossible. Patients who have experienced one of these attacks say it feels like drowning or choking.

Anthony Montanaro, head of the Division of Allergy and Clinical Immunology at Oregon Health and Science University in Portland, says asthma is not one disease
The majority of asthma sufferers can stave off flare-ups with daily medicines that reduce inflammation and combat allergies. But for some individuals, the attacks keep coming, despite these therapies. According to the World Health Organization, about 235 million people worldwide suffer from asthma. Experts estimate that 20% of those individuals have severe asthma, and the disease is poorly controlled in a further 20% of that group.
For some severe asthmatics, the standard therapies simply do not work well. That is because asthma is not one disease, it is many. “There are no two asthmatics that are exactly the same,” says Anthony Montanaro, head of the Division of Allergy and Clinical Immunology at Oregon Health and Science University in Portland.
There are no two asthmatics that are exactly the same
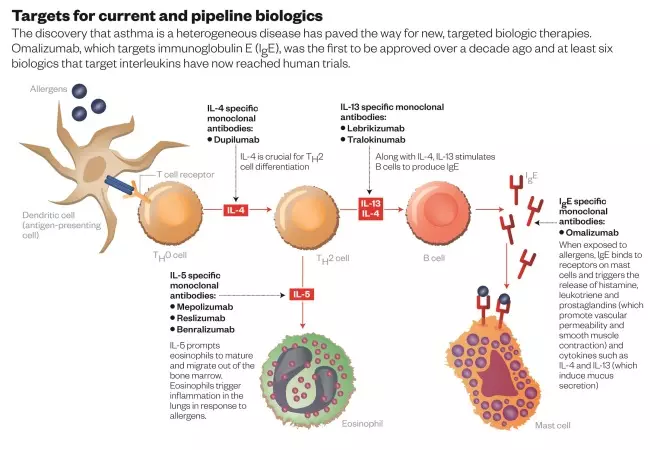
The realisation that asthma comes in many different forms has paved the way for new, targeted biologic therapies. More than a decade ago, Australia approved the first, omalizumab, developed by Basel, Switzerland-based Roche for patients with poorly controlled allergic asthma. But there are a wealth of new therapies on the horizon. Those closest to market are monoclonal antibodies — identical ‘designer’ antibodies produced in the lab — that block certain interleukins, cell-signalling molecules that seem to play a crucial role in some forms of asthma (see ‘Targets for current and pipeline biologics’).
These medicines will not work for all asthma sufferers; they are aimed at a small group of patients who have specific biological markers and who do not respond to standard therapies. But Anna Murphy, a consultant respiratory pharmacist at University Hospitals of Leicester NHS Trust, UK, thinks they could still have a significant impact. “It’s the next step toward personalised treatment,” she says. “I love the thought of being able to say, ‘Let’s find out what’s driving this patient’s asthma, then we can match the right treatment for that patient.’ Hopefully we can really improve outcomes for our patients and prevent death.”
Turning things around
At least six interleukin inhibitors have reached human trials for asthma, and two are awaiting regulatory approval. But the future of these medicines did not always look so bright. When researchers first tested London-based GlaxoSmithKline’s antibody mepoluzimab in a human efficacy trial in the late 1990s, it flopped[1]
. Other inhibitors suffered a similar fate. “The first few studies that came out were actually quite negative,” Murphy says. The results were so disappointing, in fact, that GlaxoSmithKline shelved mepoluzimab, and Schering Plough (since taken over by Merck, headquartered in Kenilworth, New Jersey) terminated development of its compound, called reslizumab, and eventually sold the rights to another company.
Both mepolizumab and reslizumab are designed to block a molecule called interleukin-5 (IL-5) by binding to it and preventing it from attaching to its receptor. IL-5 prompts immune cells called eosinophils to mature and migrate out of the bone marrow. Eosinophils protect against infections, but they can also trigger inflammation in the lungs in response to allergens. Asthma researchers reasoned that if they could block IL-5, they might be able to reduce the number of eosinophils and eliminate some of the symptoms of asthma. “They figured that if IL-5 is present in one asthmatic, it’s going to be present in all asthmatics,” says Jonathan Corren, an allergy specialist at the Ronald Reagan University of California Los Angeles Medical Center. But the initial efficacy trials failed to take the diversity of asthma into account. Not all individuals with asthma have a disease driven by IL-5.
Pharmaceutical companies now recognise that “you’ve got to do the clinical trial in the person with the right biology”, as Liam Heaney, an asthma researcher at Queens University Belfast who specialises in hard-to-treat asthma, puts it. That became clear in 2009, when two studies in the New England Journal of Medicine showed that mepolizumab significantly reduced the number of severe asthma attacks in a small group of sufferers who had high levels of eosinophils despite corticosteroid treatment[2]
[3]
. The problem with the initial studies was not that mepolizumab did not work, it was that the investigators had not tested it in the right group of patients.
You’ve got to do the clinical trial in the person with the right biology
In November 2014, off the back of a couple of phase III clinical trials, GlaxoSmithKline filed for regulatory approval of mepoluzimab in the United States and Europe. The trials looked at asthmatics with the same biomarker as had yielded the earlier promising results. One showed that a monthly subcutaneous injection of mepoluzimab reduces the frequency of asthma exacerbations by 53% compared with placebo[4]
. A second study found that mepoluzimab allowed asthma sufferers to reduce their daily dose of oral corticosteroids, yet still control their asthma[5]
. In June 2015, a US Food and Drug Administration (FDA) advisory committee voted unanimously to recommend mepolizumab for regulatory approval in adults.
Two other companies are pursuing the same target. Teva Pharmaceutical Industries, based in Petah Tikvah in Israel, presented phase III results for its IL-5 blocker, reslizumab, in September 2014. The studies showed that a monthly injection of reslizumab reduced the number of asthma exacerbations compared with placebo by 50% to 60% in people with inadequately controlled asthma and high eosinophils[6]
. The company filed an application with the FDA in 2015, and expects a decision by March 2016.
Benralizumab, developed by London-headquartered AstraZeneca, is aimed at the same subgroup of patients but takes a slightly different approach, blocking the IL-5 receptor rather than IL-5 itself. The phase III results have not yet been announced, but a phase II trial showed that a 100mg dose not only reduced exacerbations, but also improved lung function and asthma control[7]
.
Drug companies also have their sights set on other targets, primarily two other interleukins, IL-13 and IL-4. These molecules have similar effects: they help eosinophils infiltrate lung tissue, prompt epithelial cells to become mucus-producing cells, and contribute to stiffening of the airways.
Both Roche and AstraZeneca are developing anti-IL-13 therapies. In March 2014, Roche announced that its offering, the monoclonal antibody lebrikizumab, had fared well in a phase II study. The researchers divided the study population by their levels of periostin, a protein produced by airway epithelial cells that is induced by IL-13. Those with high periostin experienced a 60% reduction in asthma attacks. Those with low levels only experienced a 5% reduction.
Dupilumab, a therapy being developed by Regeneron and Sanofi, targets the shared IL-13/IL-4 receptor. In May 2015, the companies announced results from a phase II study at the American Thoracic Society International Conference. The two highest doses of dupilumab significantly improved lung function in patients with high eosinophils. Individuals taking dupilumab also experienced fewer asthma attacks.
“Over the last three years there has been a turnaround,” says Marc Rothenberg, director of the Division of Allergy & Immunology at Cincinnati Children’s Hospital Medical Center in Ohio. As he sees it, these once-disappointing therapies now show enormous potential. “These drugs can have a huge impact”.
However, Montanaro is less enthusiastic about the new therapies. “You do see significant differences in the rate of exacerbations, but this is far from a cure,” he says. “Ideally you would like to develop a biologic that would replace the need for any medications.”
You do see significant differences in the rate of exacerbations, but this is far from a cure
Costly endeavour
As a long-term treatment, biologics are an expensive option. Omalizumab costs about US$10,000 a year. Barclays analysts predict that mepolizumab, if approved, could sell for as much as $15,000 a year. The high price tag means that doctors will reserve these medicines for patients with severe asthma who have failed other therapies.
And not everyone is convinced that the benefit justifies the cost. In a comment in the New England Journal of Medicine, Parameswaran Nair, a respirologist at McMaster University in Hamilton, Ontario, notes that poor asthma control is often the result of poor adherence to existing medicines[8]
. He points out that even the placebo group in one of the phase III trials of mepoluzimab experienced a 50% reduction in exacerbations, and speculates that fine-tuning existing medicines and working to improve adherence might achieve the same benefit as adding a costly biologic. “Most patients in this clinical trial might have had [an] improvement in symptoms without mepolizumab simply by the institution of good clinical practice, as recommended by current international guidelines,” he writes.
However, William Busse, an asthma researcher at the University of Wisconsin in Madison, does not think the high price should be a deal breaker. “All biologics are expensive,” he points out. And interleukin blockers — at least initially — will only be given to the sickest patients, those who burden the health system most. A 2013 analysis funded by Teva estimates that, in the United States, between 2.5% and 5% of asthma patients have severe, uncontrolled disease. However, these individuals are responsible for 37.5% of total asthma-related costs, which means that each of these patients racks up, on average, between $16,000 and $32,000 in asthma-related medical bills each year. So it is at least possible that the interleukin blockers will save money.
Looking ahead
Although the new biologics are aimed at people with severe, uncontrolled asthma, they will not work for all patients in this group. But sorting out who will benefit and who will not could be difficult. Heaney, who is heading up the UK Refractory Asthma Stratification Programme (RASP), echoes Nair’s point that some patients have poorly controlled asthma because they are not taking their medicines correctly. Identifying those patients is important, Murphy says. “In our healthcare system, we can’t justify spending £1,000 a month compared to £20 for an inhaler.”
In our healthcare system, we can’t justify spending £1,000 a month compared to £20 for an inhaler
There is another subgroup that Heaney hopes to identify through RASP — patients with severe asthma who are taking their medicines correctly, but whose disease is not characterised by high eosinophil or periostin levels. These individuals are unlikely to benefit from interleukin inhibitors.
Even within the subgroup of asthma sufferers who have the right biomarker profile, physicians may have difficulty determining which patients should receive which antibody. The biomarkers linked to each medicine tend to go up and down together. So a patient might have a high eosinophil count in addition to high periostin levels. “Should they get a anti-IL-5 or an anti-IL-13,” Heaney asks. “We don’t absolutely know.”
Busse speculates that the new antibody therapies could also play a role in asthma prevention. Young children may show signs that they are at risk for developing asthma — for example, wheezing during respiratory infections. Busse wonders what might happen if doctors gave these high-risk children anti-interleukins during this vulnerable period. “Could you prevent the progression of the disease and maybe prevent its full expression at a later date,” he muses. “This is theory. It hasn’t been tested yet,” he says, but it seems possible. “I think the use of biologics will be more than just for severe disease.”
References
[1] Leckie MJ, ten Brinke A, Khan J et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. The Lancet 2000;356:2144–2148.
[2] Nair P, Pizzichini MM, Kjarsgaard M et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. New England Journal of Medicine 2009;360:985–993.
[3] Haldar P, Brightling CE, Hargadon BN et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. New England Journal of Medicine 2009;360:973–984.
[4] Ortega HG, Liu MC, Pavord IDet al. Mepolizumab treatment in patients with severe eosinophilic asthma. New England Journal of Medicine 2014;371:1198–1207.
[5] Bel EH, Wenzel SE, Thompson PJ et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. New England Journal of Medicine 2014;371:1189–1197.
[6] Castro M, Zangrilli J, Wechsler ME et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. The Lancet Respiratory Medicine 2015;3:355–366.
[7] Castro M, Wenzel SE, Bleecker ER et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. The Lancet Respiratory Medicine 2014;2:879–890.
[8] Nair P. Anti-interleukin-5 monoclonal antibody to treat severe eosinophilic asthma. New England Journal of Medicine 2014;371:1249–1251.
You may also be interested in

Helping respiratory patients breathe easy

Asthma: long-term management