Courtesy of Colorado School of Mines
The much-heralded ‘precision medicine revolution’ promises tailored treatments based on individual genetic profiles. But there are other aspects of medicine that could also benefit from a more personalised approach, such as the ability to accurately monitor drug levels and biological molecules inside patients in real time.
In vivo implantable biosensors could allow for this. A variety of biosensors are already in development, promising a broad range of clinical applications. They could, for example, trace inflammation by sensing increased nitric oxide levels or provide individualised pharmacokinetic data for bespoke dosing of antibiotics and cancer drugs. “Imagine the power of being able to have a patient sit in a chair and, as we are infusing the drugs, we will have a [device] that is sensing different drugs and adjusting dosages as a function of the patient’s responses,” says Netzahualcóyotl Arroyo-Currás, who is in the process of setting up his own sensor group at Johns Hopkins University, Baltimore, Maryland.
Ambitions for such technologies are high, but the development is not quite there yet. Scientists are still working to produce selective, sensitive, reversible and biocompatible sensors.
Biosensors in diabetes care
Perhaps unsurprisingly, the most advanced in vivo sensor systems are already being used for continuous glucose monitoring (CGM). CGM systems have been available on the NHS since 2017 for some people living with type 1 diabetes mellitus — even Theresa May, the UK prime minister, has been photographed wearing one — and trials have found the devices successful in reducing the incidence of hypoglycaemia. The devices use electrochemical sensors that are inserted just under the skin; they work for around 14 days, measuring glucose levels in the interstitial fluid. The sensors use the enzyme glucose oxidase to oxidise glucose, forming hydrogen peroxide which reacts with platinum inside the sensor, generating a concentration-dependent electrical signal that can be sent wirelessly to an associated device, such as a smartphone or other sensor.
The aim for most of the newer real-time glucose monitoring technologies is to find smaller sensors that will work for longer, reducing the need for continuous implantation procedures. One potential avenue of research is exploring optical methods; the first light-based CGM system, Eversense® (Senseonics), was approved by the US Food and Drug Administration in June 2018 and can be worn for 90 days. The sensor is coated with a fluorescent chemical that produces light instead of an electrical signal; the amount of light is measured and reported to a smartphone app.
Biosensors in development
Here are four promising biosensor technologies that are in development.
Ionophore sensors
Kevin Cash, a chemical engineer at the Colorado School of Mines in Golden, Colorado, and colleagues have designed a number of sensors that measure optical or fluorescent signals. Cash started developing optical sensors, known as optodes, in Heather Clark’s laboratory at Northeastern University, Boston, Massachusetts. The optical signal is detected simply by photographing the surface of the skin to capture an image of the light produced in vivo. “Basically, you take a picture of it to be able to read out what’s going on in the body,” explains Cash.
The ionophore-based optical sensors are much smaller than a human hair and each one of these can change colour when the concentration of the drug, or the ion or the metabolite, changes
Clark, a chemical biologist at Northeastern University, and her team have developed a modular family of sensors approximately 100nm in size, enclosed in a biocompatible polymer coating. One of the team’s earliest successes in this area was a sensor built in 2013, which tracked histamine levels in vivo using the enzyme diamine oxidase and a phosphorescent oxygen nanosensor. The histamine is broken down by diamine oxidase, causing a reduction in local oxygen levels which, in turn, increases the phosphorescence of the oxygen-sensitive nanosensor[1]
.

Source: Courtesy of Colorado School of Mines
Kevin Cash, a chemical engineer at the Colorado School of Mines in Golden, Colorado, calls his ionophore-based optical sensors ‘nanoparticle tattoos’ because they sit in the skin layers
Cash calls his ionophore-based optical sensors ‘nanoparticle tattoos’ because they sit in the skin layers. “These are much, much smaller than a human hair and we can make each one of these change colour when the concentration of the drug, or the ion or the metabolite, changes,” he explains. “We put a bunch of them into a little injection site so its looks like a tiny tattoo, almost — a little circle — and as that changes colour or other optical properties, we can report out what is going on inside the blood stream.”
These sensors can detect ions such as lithium, potassium, sodium and calcium — all of which are commonly measured in standard metabolic blood screening. Although the sensors measure the interstitial concentrations of these ions and other small molecules, rather than their concentration in the blood stream, the two values are proportional.
The nanoparticle tattoos are described by Cash as a tiny “squishy, rubber bouncy ball” made from a polymer capsule encasing each sensor. The sensor consists of a pH indicator (chromoionophore), an analyte-specific ligand (ionophore) and a charge-balancing additive. The positively charged ion of interest is pulled into the nanoparticle, causing deprotonation of the pH indicator molecule and a minute colour change which is probably invisible to the naked eye.
The sensor works quickly — the speed at which the image can be captured is the biggest temporal limitation. “For a lot of the work we do, we image once a minute or so, just because otherwise you get too much data,” says Cash. “For in vivo imaging, not many things outside of the nervous system change all that fast,” he adds.
Carbon nanotube sensors
Another approach is carbon nanotube sensors. Single-walled carbon nanotubes were discovered in 1993 and have unique optical properties. They fluoresce in the near-infrared and do not photo-bleach — meaning they do not lose their ability to fluoresce.
Near-infrared is the perfect spectral range for in vivo work because you can see deeper into tissue
Michael Strano, chemical engineer at Massachusetts Institute of Technology in Cambridge, Massachusetts, and many of his former group members are now independently designing their own carbon nanotube devices, for example, researcher Gili Bisker, who will soon move to Tel Aviv University, Israel. “The wavelengths at which [carbon nanotubes] emit are longer than visible wavelengths, so we cannot see them with the naked eye,” says Bisker. “However, this is the perfect spectral range for in vivo work because at this part of the spectrum both blood and water do not absorb much, so you can see deeper into tissue,” she adds.

Source: Courtesy of Gili Bisker
Gili Bisker, a researcher who will soon move to Tel Aviv University, Israel, is independently designing her own carbon nanotube sensors
To create the sensor, Strano devised the corona phase molecular recognition (known as ‘CoPhMoRe’) concept, where the nanotube is wrapped with a functionalised polymer. “The specific conformation that the polymer adopts when it is wrapped around the carbon nanotube is what enables the detection of the analyte of interest, because it forms a unique 3D structure that allows the analyte to bind specifically and selectively to the surface of the nanotube,” explains Bisker. From the modulation of the intensity or the wavelength of the fluorescence you can infer the local concentration of the analyte.
As with other optical sensors, the mm-sized device is implanted subcutaneously. It is first encapsulated within a hydrogel that does not disrupt the sensor’s responsiveness. The devices have been shown to work successfully in mice for up to 300 days, giving hope for long-term sensing systems that would not need to be constantly replaced. Sensors have been designed to track nitric oxide, providing an indication of inflammation within the body, and other detectable molecules, including insulin, dopamine, L-ascorbic acid, riboflavin, L-thyroxine and oestradiol. Bisker has also investigated detection of larger protein molecules and created a system that can monitor fibrinogen — the protein building block used in blood clots[2]
.
We are still trying to convince researchers and, in my experience, at least a third of the researchers I run into still think carbon nanotubes are toxic
But one of the major drawbacks with this approach is concern over the toxicity of carbon nanotubes in humans. Questions were first raised over this issue in around 2006, when research indicated that the elongated shape of carbon nanotubes and their hydrophobic nature could trigger similar toxic responses to those caused by asbestos particles when inhaled. Since then, it has been shown that when the nanotubes surfaces are chemically modified this toxicity can be prevented[3]
. “We have shown that our carbon nanotubes sensors [can be made non-toxic] as well,” says former Strano group member Nicole Iverson, now at the University of Nebraska–Lincoln. “Right now, we are still trying to convince researchers and, in my experience, at least a third of the researchers I run into still think carbon nanotubes are toxic.”
Another problem with optical sensing methods is that the signal is usually read by an external device. “We can only really look at things basically in the upper layers of the skin. It doesn’t work well if we wanted to look specifically in the liver or in the kidney,” explains Cash.
Opto-acoustic sensors
One alternative method that might get round this is opto-acoustic sensors. Opto-acoustics can provide similar imaging capabilities to ultrasound, but rather than sound-in and sound-out, a light signal is used to create very small but detectable sound waves. Because the sound is not absorbed as readily as light, information from deeper tissue can be retrieved.
Haemoglobin produces a different opto-acoustic response when oxygenated, revealing areas of the body that are hypoxic
Jesse Jokerst, a nanoengineer at the University of California San Diego, has been creating sensors based on opto-acoustics. “The mechanism is essentially thermal expansion,” he explains. “When light propagates through the body and is absorbed by the target tissue, there is a very short and fast heating event, and that heating event causes swelling. The pressure wave that is created when the target tissue expands can then be detected as sound.” The short, 5ns pulses can be detected by the same kind of transducer used in regular ultrasound, but the contrast in how different tissues respond is much greater, making it possible to gain chemical information at a frame rate of about five images per second. The classic example is haemoglobin, which produces a different opto-acoustic response when oxygenated, revealing areas of the body that are hypoxic.

Source: Courtesy of Jesse Jokerst
Jesse Jokerst, a nanoengineer at the University of California San Diego, has been creating sensors based on opto-acoustics
Jokerst has devised a smart catheter to monitor levels of the anticoagulant heparin in patients (see Figure 1)[4]
. About 500 million doses of heparin are administered globally each year, but it is notoriously difficult to manage because the concentration window is narrow, particularly for children. Incorrect dosages lead to haemorrhages or clotting. Jokerst has coated a catheter with the small, clinically approved molecule methylene blue (methylthioninium chloride), which binds to heparin and dramatically increases its photo-acoustic signal. The idea is to place this catheter into a patient’s vein. “The patient would wear a flexible ultrasound transducer over there arm where the [intravenous catheter] is placed, and use that for continuous monitoring of heparin as opposed to just doing point measurement every 12 to 24 hours,” says Jokerst. He has tested the device in principle using samples from 28 patients, which showed good correlation with the ‘gold standard’ blood test.
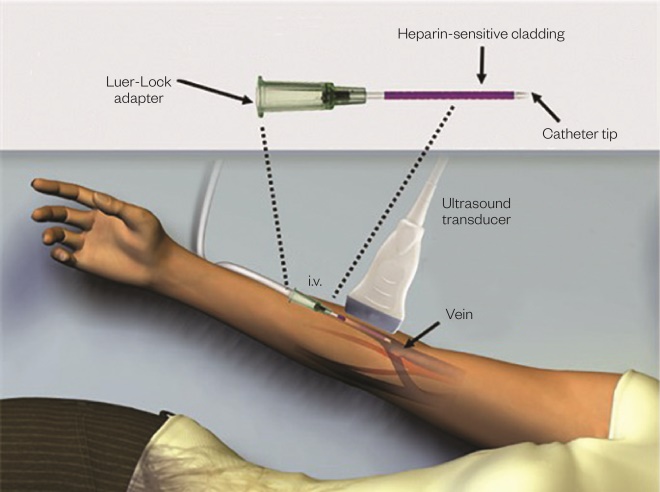
Figure 1: Opto-acoustic sensor
Source: Courtesy of Jesse Jokerst
A catheter is coated with the small, clinically approved molecule methylene blue (methylthioninium chloride), which binds to heparin and dramatically increases its photo-acoustic signal, allowing levels to be monitored continuously via a flexible ultrasound transducer over the arm.
Ultimately, Jokerst is aiming for a complete system. “What would be really fantastic is if we could have the signal from the catheter feed back into an infusion pump, so it would start to become a closed system.” He is confident that wearable photo-acoustic in vivo sensors will become a reality, although the current need for a laser to produce the light pulses that create the signal makes the equipment large and expensive. “One thing we have recently reported is that we can use LEDs, rather than a laser, to produce the photo-acoustic signal and so that starts to not only reduce the size of the device but it also reduces the price,” says Jokerst.
Aptamer sensors
For most of the systems, there is a long development process for each sensor, but the ‘holy grail’ would be a ‘plug and play’ system that can be modified to sense different drugs or biomolecules with no additional reagents needed.
Kevin Plaxco, a chemist from University of California Santa Barbara, thinks he may have such a system, based on aptamers — single strands of DNA or RNA, usually 30–80 nucleotides in length with a variable 40-nucleotide region — designed to bind a specific target. Arroyo-Currás, who used to work with Plaxco, explains: “[These sensors] look like wires and are made of gold, which we modify with a single strand of DNA with a redox reporter [such as methelyne blue attached at its other end]. On binding [to the analyte], the aptamer reorganises itself to wrap around the analyte of interest” (see Figure 2).
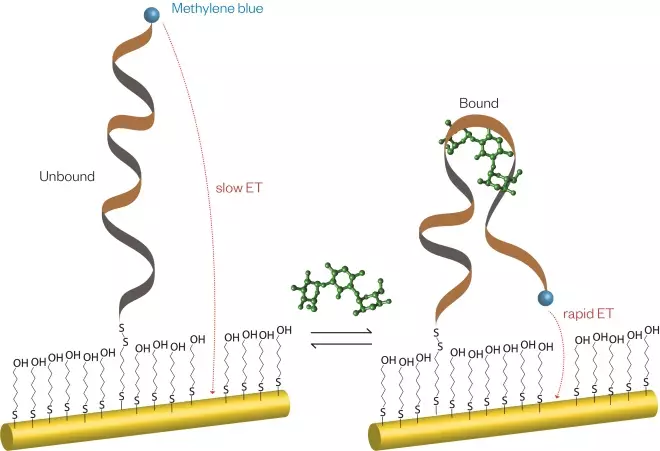
Figure 2: Aptamer sensor
Source: Courtesy of Netz Arroyo
A single strand of DNA on a gold wire can detect a large variety of analytes with no additional chemical reagents. On binding an analyte, the DNA molecule changes its conformation, which in turn changes the efficiency of electron transfer between the gold wire and methylene blue ‘redox reporter’ molecule attached to the DNA molecule.
The redox reporter molecule allows electrons to efficiently transfer to the gold wire electrode, but the large conformational change in the aptamer molecule affects the distance between the reporter and electrode, and therefore the size of this signal can be used to calculate the analyte concentration, with no other chemical reactions involved.
Aptamers offer the ability to record full pharmacokinetic profiles over hours, sampling once every two seconds
Arroyo-Currás explains that the new system could prove more versatile: “The limitation of the approach that we currently use in glucose sensors is that you need an enzyme that can metabolise the target that you want to measure,” he says. “We want to move away from that.” The aptamer sensor and accompanying electrodes make a device comparable in size to a human hair and this is implantable, but is also small enough to be injected in a catheter within a vein. This gives the ability to record full pharmacokinetic profiles over hours, sampling once every two seconds.
One area Plaxco’s group has investigated is in vivo levels of aminoglycoside antibiotics. In animal experiments, Arroyo-Currás and Plaxco coupled a sensor for tombramycin to a drug delivery system and were able to regulate the drug’s concentration in the animal’s plasma, dosing eight times per minute[5]
. They were also able to show that the speed of distribution and clearance of the drug varied within and between animals. The drug distribution was largely dependent on the blood and body volumes, but differed much less as a function of time between animals. However, the clearance rate was more variable, being dependent on kidney function, which tended to slow over time due to differences in blood pressure and hydration levels.
So far the sensors have only been tested in mice. “If you give us another 10 years, it is likely that we will find at least a few applications in humans,” says Arroyo-Currás. Other systems may make it quicker and many biosensor researchers see it as only a matter of time before this sort of monitoring becomes an everyday reality in medicine. “I envision for diseases like diabetes, 100% of patients should be wearing these sorts of things,” says Cash. There are also likely to be applications in tracking chemotherapy progress and providing bespoke dosing for individual cancer patients.
Given the array of different technologies on offer for in vivo sensing, it seems certain that we will soon be seeing these devices in clinical use — both in diagnostics (see Panel) and as part of therapeutic protocols. Having small wearable devices that can provide real-time data and automatic dosing will represent a real advance for many patients. Whether one technology eventually dominates remains to be seen. “I think getting the price-point down is going to be the key to translation,” says Jokerst, “but I am optimistic there will continue to be slow but steady progress.”
Panel: How biosensors could improve diagnosis
In vivo sensing also has potential for diagnostics and these types of devices could become the first port of call in emergency or intensive care units, providing much more information than current diagnostics, and at speed. “Instead of just measuring 1 or 2 things, you are measuring 15 things that are all important for the clinician to know,” says Kevin Cash, a chemical engineer at the Colorado School of Mines in Golden, Colorado. “We now have this ability to collect this valuable data on a continuous time horizon and put that to use to make better and faster decisions.”
In vivo diagnostic biosensors of a different type are also on their way and could limit the need for invasive biopsies. Gabriel Kwong, at Georgia Institute of Technology in Atlanta, Georgia, set up the company Glympse Inc in 2016 to develop diagnostics based on what he calls “synthetic biomarkers”[6]
.
“The challenge with a naturally shed biomarker is that it’s not very sensitive at all,” says Kwong. “As they are shed into the blood they are diluted by a tremendous amount.”
The system Kwong is developing uses nanoparticles containing peptides that can be cleaved by specific disease-associated proteases. The nanoparticles are injected into a patient and, when they arrive at the diseased microenvironment, the local disease-associated protease will release small peptide fragments of a specific mass that act as the reporter. These can be collected in urine for detection by mass spectrometry. “Our advantage is we can do diagnosis at a much, much earlier point,” says Kwong. Any response is amplified as a single protease can cleave hundreds of peptides in these nanoparticles per hour.
Studies in mice have shown that it is possible to use this method to non-invasively monitor liver fibrosis (a wound-healing response to chronic liver injury), and for detection of early-stage colorectal cancer (detecting the disease before it shows up in secreted blood biomarkers). Kwong says that the method can profile the progress of any disease rather than just providing the static snapshot that a biopsy offers. “With our approach, because we are looking at enzymatic activity, the activity of the cells is actually predictive of where that disease is going.” Kwong hopes to use his ‘imaging-free’ diagnostics in patients by 2019.
References
[1] Cash KJ & Clark HA. Phosphorescent nanosensors for in vivo tracking of histamine levels. Anal Chem 2013;85(13):6312–6318. doi: 10.1021/ac400575u
[2] Bisker G, Dong J, Park HD et al. Protein-targeted corona phase molecular recognition. Nat Commun 2016;7:10241. doi: 10.1038/ncomms10241
[3] Ali-Boucetta H, Nunes A, Sainz R et al. Asbestos-like pathogenicity of long carbon nanotubes alleviated by chemical functionalization. Angew Chem Int Ed Engl 2013;52(8):2274-2278. doi: 10.1002/anie.201207664
[4] Wang J, Chen F, Arconada-Alvarez SJ et al. A nanoscale tool for photoacoustic-based measurements of clotting time and therapeutic drug monitoring of heparin. Nano Lett 2016;16(10):6265-6271. doi: 10.1021/acs.nanolett.6b02557
[5] Arroyo-Currás N, Somerson J, Vieira PA et al. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc Natl Acad Sci USA 2017;114(4):645–650. doi:10.1073/pnas.1613458114
[6] Kwong GA, von Maltzahn G, Murugappan G et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotechnol 2013;31(1):63-70. doi:10.1038/nbt.2464