Claire Standage
When David Alldred, a researcher in medicines use and safety at the University of Leeds, watched his tweet about deprescribing gather more than 270,000 impressions, 3,600 likes and 1,100 retweets, he knew he had struck a chord with prescribers, patients and the public.
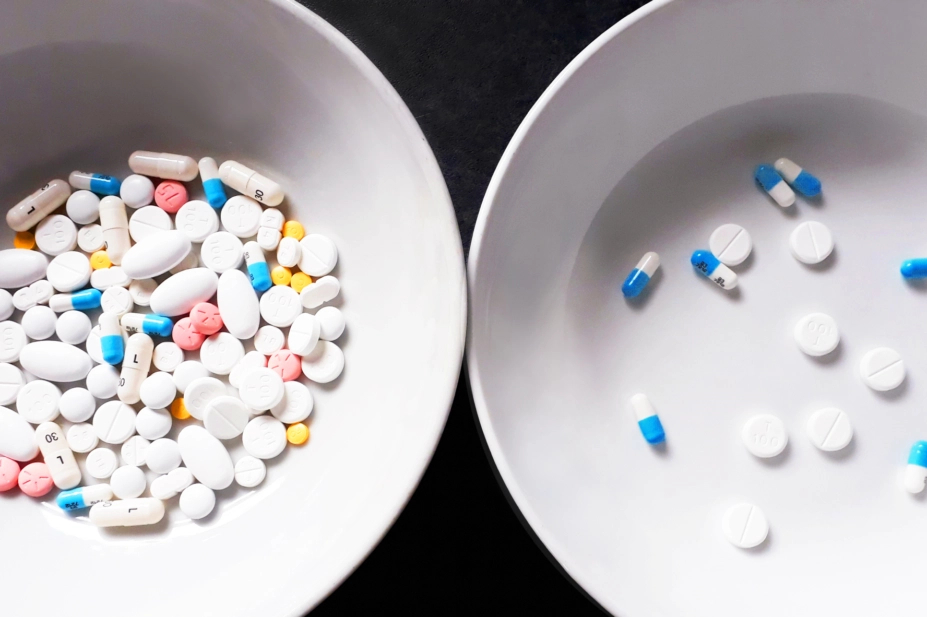
The tweet was about a medication review with a 92-year-old patient, who was prescribed nine medicines per day but was not taking any of them because of a fear of side effects.
“The pharmacist talked to the patient … and they agreed, along with the patient’s GP, to reduce the medicines to two per day,” explains Alldred. This dropped the weekly tablet burden from 77 to 14, creating the striking image tweeted by Alldred, which sparked an international debate about deprescribing.
The Twitter debate reflects worldwide interest in deprescribing, which is rapidly gathering momentum. Deprescribing networks already exist in Canada and Australia, and a network is in the process of being created in England too. This drive to raise the profile of deprescribing comes in response to increasing levels of polypharmacy, which have prompted national policies in England, Scotland and Wales, as well as the first piece of National Institute for Health and Care Excellence (NICE) guidance on multimorbidity and polypharmacy in 2017.

Source: Courtesy of David Alldred
David Alldred, a researcher in medicines use and safety at the University of Leeds, says that one of the reasons why polypharmacy has become so prevalent is the multitude of clinical guidelines that are based on single conditions
Although polypharmacy can be appropriate, it may also be problematic, contributing to adverse drug reactions, falls, cognitive impairment, noncompliance, admission to hospital and death. The number of older people in England who are taking five or more prescription or over-the-counter medicines has quadrupled over the past two decades, from 12% to 49%[1]
. However, this is not a situation that is unique to England — research from the United States and Australia indicates similar levels of polypharmacy among older people, with the number of medicines used increasing with age[2],[3]
.
It’s very difficult when you’re faced with a patient who has multiple conditions and multiple medicines: which ones do you target for deprescribing?
One of the reasons why polypharmacy has become so prevalent is the multitude of clinical guidelines based on single conditions, explains Alldred. For a patient with asthma, diabetes and rheumatoid arthritis, each guideline recommends perhaps three or four medicines, so the patient can quickly be taking ten or more medicines. “It’s very difficult when you’re faced with a patient who has multiple conditions and multiple medicines: which ones do you target for deprescribing?”
Breaking down barriers
“Practitioners don’t always know which medicines cause harm,” says Duncan Petty, who splits his time between medicines optimisation research at the University of Bradford and his work in primary care doing medication reviews within general practice. “We have some evidence for individual medicines … but there is very little evidence correlating combinations of medicines or polypharmacy with outcomes such as hospitalisation or falls.”

Source: Courtesy of Duncan Petty
Duncan Petty, who splits his time between medicines optimisation research at the University of Bradford and his work in primary care, says that practitioners do not always know which medicines cause harm
We have some evidence for individual medicines … but there is very little evidence correlating combinations of medicines or polypharmacy with outcomes such as hospitalisation or falls
Petty’s work focuses on frail older people. Many of the patients he sees have already made the decision to stop their medicines. “When we visit someone at their house, it’s full of medicines,” he says. “So, it’s about helping them safely stop the ones they no longer need and following them up to check that they have continued to remain safe.”
To do this, practitioners need to understand the barriers to deprescribing, which include lack of awareness, inertia, fear of negative consequences (see Panel 1) and the complexities and workload implications of stopping a medicine, especially if it does not appear to be causing any harm.
Panel 1: Legal considerations
Órla Kelly, a pharmacist and partner at Cantillons Solicitors in Cork, Ireland, acknowledges that prescribers may have concerns about deprescribing. “From a legal perspective, you’re held to the test of a reasonable practitioner.” This means being compared with your peers and asking whether would they have done the same.
A lack of evidence or guidance is not necessarily a barrier to deprescribing, says Kelly, and should not be used as a get-out clause. “You don’t need to do anything differently with deprescribing; you just need to prescribe reasonably and appropriately in accordance with the guidelines, if they’re there.”
Kelly highlights the importance of informed consent. “You need to tell the patient about alternatives and you need to tailor [your advice] to the person.” This means helping the patient understand what might happen if a medicine is stopped and whether any consequences could be harmful.
“Document the decision; document the thought process,” says Kelly. “It’s OK to go outside the box: if there is a guideline, be aware and apply it or state why you’re not applying it.”
Kelly adds that prescribers must also consider the legal risks of continuing to prescribe an inappropriate medicine. “You don’t want to be the outlier, not [deprescribing]. That’s equally risky.”
Patients may have an attachment to their tablets, especially if they were told that they would need to take them for life. “The more hierarchical the person is who told them, the less likely they will want to give it up, even when we tell them that the evidence has changed or about the potential harm,” says Petty.

Source: Courtesy of Nina Barnett
Nina Barnett, consultant pharmacist at NHS Specialist Pharmacy Service and London North West University Healthcare NHS Trust, believes that many of the barriers to deprescribing can be overcome by ensuring consultations with patients use a shared decision-making approach
Nina Barnett, consultant pharmacist at NHS Specialist Pharmacy Service and London North West University Healthcare NHS Trust, says clinicians are sometimes anxious about how patients will react if they broach the subject of deprescribing. Patients may be concerned that the healthcare system cannot afford to treat them or, because they are old, they are not worth the resource. They may also question why the medicine was prescribed in the first place.
If it takes three or four years for a medicine to have any benefit and someone has a life expectancy of a year, the patient is not going to benefit and you’re potentially putting them at risk
Prescribers need to consider life expectancy, which can be hard to predict and even harder to talk about. “A lot of medicines take time for benefit to accrue,” says Alldred. “If it takes three or four years for a medicine to have any benefit and someone has a life expectancy of a year, the patient is not going to benefit and you’re potentially putting them at risk.”
Clinicians can also find it hard to explain the benefits and risks of taking a medicine. “Being able to interpret the evidence base is a real challenge,” says Alldred, particularly for older patients where the evidence is lacking. “We need to be honest with patients about that and say ‘we don’t really know what the benefits are in your age group’.”
To address some of these challenges, Alldred’s team is studying pharmacist prescribing in care homes. As part of the study, pharmacists will develop pharmaceutical care plans and identify medicines that are no longer needed or where the risk-benefit profile is unfavourable. “If it’s shown that pharmacists are effective in that prescribing role in care homes then that’s going to have a potential major impact on practice and policy,” says Alldred, who expects results in 2020.
Why the medicine was prescribed
Knowing why a medicine was prescribed is an important enabler for deprescribing, according to Barbara Farrell, a clinical pharmacist at Bruyère Geriatric Day Hospital in Ottawa, Canada, and a research scientist at the Bruyère Research Institute.

Source: Courtesy of Barbara Farrell
Barbara Farrell, a clinical pharmacist at Bruyère Geriatric Day Hospital in Ottawa, Canada, and a research scientist at the Bruyère Research Institute, has developed deprescribing guidelines using the same approach that people would use to develop a prescribing guideline
Farrell chairs the healthcare education provider committee for the Canadian Deprescribing Network (CaDeN), which is calling for clinicians to document the reason for a prescribed medicine and the intended duration. In Canada, similar to the United States, patients typically receive healthcare from several unconnected providers. “You don’t have their notes and the patient doesn’t remember why [the medicine] was prescribed. So, you’re kind of nervous to stop it because you don’t know why they are taking it,” explains Farrell.
“We’re luckier in the UK,” says Petty. “Most patient information ends up back in the primary care system.” For some patients, it may be necessary to go back to the paper notes. “You can usually find an answer in a letter or in the narrative somewhere,” says Petty. “I have found medicines that were started years ago because of a typing error in a letter.”
The need to search through patient records highlights another barrier to deprescribing – the resources involved. Clinicians need to scour medical notes, consult with the patient, come up with a plan and then provide support and follow-up. “It is very time consuming to do medication reviews properly,” says Petty. “At least half an hour to 45 minutes.”
Alldred adds that more complex cases, for example, patients who have been prescribed opioids or benzodiazepines, will not be one-off consultations. “It’s going to take proper investment in time and resources to be able to do that. There are no quick fixes.”
Shared-decision making
Barnett believes that many of the barriers to deprescribing can be overcome by ensuring consultations with patients use a shared decision-making approach.
She gives statins as an example and suggests that prescribers open up the conversation in this way: Your risk of having a heart attack or a stroke now is high enough that I would like to consider offering you a medicine to reduce that risk. What have you heard about statins?
“Before you’ve said anything about prescribing them you’ve used the ‘consider’ word,” says Barnett, who has developed guidance on patient-centred consultations[4]
. Some people will accept your advice, others will raise concerns – perhaps they have heard that statins cure cancer or that they kill you, she adds. “Those are two incredibly different starting places for the same medication,” says Barnett. “You’re already saying to the person that this is a negotiation, this is not a rule.” The conversation can then move to discussing a trial period with further monitoring to check whether the medicine suits the patient and remains suitable.
We’ve found that older adults and their caregivers are quite open to the idea of deprescribing but do want to know why the medication should be stopped
Emily Reeve, a researcher at the University of Sydney, Australia, is the author of a study exploring the attitudes of older patients to deprescribing, published in October 2018[5]
. She advises clinicians to discuss deprescribing with patients regularly and to incorporate reviews of appropriateness as part of normal care. “We’ve found that older adults and their caregivers are quite open to the idea of deprescribing but do want to know why the medication should be stopped, and also know that they will be supported during the process.”

Source: Courtesy of Emily Reeve
Emily Reeve, a researcher at the University of Sydney, Australia, suggests that the decision to deprescribe needs to be weighed equally against the decision to continue
Reeve suggests that the decision to deprescribe needs to be weighed equally against the decision to continue. “Continuation shouldn’t be seen as a passive or default action,” she adds.
Farrell agrees that shared decision-making is important: “Gather information about that patient’s experience of the medication, provide information in an unbiased way, explain options to the patient and find out what [options] they are interested in,” she says.
Farrell says that prescribers should take time to explain why they are prescribing a drug, how long the patient will need to take it for and if there is a plan for gradual discontinuation as the patient gets older. “Nobody ever says that last piece but they should be saying it for every prescription that they provide. That will set the expectation early – this isn’t a medication I need for my whole life.”
Evidence-based resources
Petty says that while theoretical deprescribing guidelines exist, there is little empirical evidence, especially from research involving patients.
Even the word ‘deprescribing’ — it’s a horrible word — we need a phrase the patients like and understand
“It’s as if we’ve decided this is what’s best for patients but we’ve never asked [them],” he says. “Even the word ‘deprescribing’ — it’s a horrible word. We need a phrase the patients like and understand.”
The STOPP/START medication review tool, which highlights medicine and condition combinations that are potentially inappropriate for frail older people, is a useful starting point, says Petty (see Panel 2). It lists medicines for which there is clear evidence. “[But] there are things missing that we just don’t know about — for instance, combinations of medicines in frailty or pure numbers of medicines in frailty,” he says. “The trickier decision in practice is one where there isn’t any direct evidence.”
Panel 2: STOPP/START medication review tool
The STOPP/START medication review tool is designed to uncover inappropriate prescribing known to cause adverse drug reactions in older people. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions) contains rules relating to the most common and potentially dangerous instances of inappropriate prescribing in this group.
START (Screening Tool to Alert doctors to the Right Treatment) contains rules relating to common instances of prescribing omission.
Example of STOPP criteria for the respiratory system include:
- Theophylline as monotherapy for chronic obstructive pulmonary disease (COPD) (there is a safer, more effective alternative; risk of adverse effects owing to narrow therapeutic index);
- Systemic corticosteroids instead of inhaled corticosteroids for maintenance therapy in moderate–severe COPD (unnecessary exposure to long-term side effects of systemic steroids);
- Nebulised ipratropium with glaucoma (may exacerbate glaucoma).
Example of START criteria for the respiratory system:
- Regular inhaled beta2 agonist or anticholinergic agent for mild-to-moderate asthma or COPD;
- Regular inhaled corticosteroid for moderate-to-severe asthma or COPD, where predicted forced expiratory volume in 1 second <50%;
- Home continuous oxygen with documented chronic type 1 respiratory failure (pO2<8.0kPa, pCO2<6.5kPa) or type 2 respiratory failure (pO2 <8.0kPa, pCO2 >6.5kPa).
Petty highlights the lack of deprescribing recommendations in national guidelines. “If you look at NICE guidance, it focuses very much on initiating medicines and monitoring them, but it would be rare to find anything about when these medicines should be stopped.”
If you look at NICE guidance, it focuses very much on initiating medicines and monitoring them, but it would be rare to find anything about when these medicines should be stopped
There have been several observational and randomised controlled trials looking at what happens when a patient’s medication is stopped: for example, the effects of stopping benzodiazepines[6]
, proton pump inhibitors[7]
and antipsychotics used in dementia[8]
. There are also studies looking more broadly at the effectiveness of medication reviews; reducing overall medication burden or reducing the number of potentially inappropriate medicines[9]
[10]
. Farrell’s team at the Bruyère Research Institute has developed an approach that allows this information to be collated to help clinicians make decisions about when and how to stop a drug. To date, they have created five deprescribing guidelines — for benzodiazepines, proton pump inhibitors, antipsychotics, antihyperglycaemics, and anticholinesterases and memantine, all of which are available via deprescribing.org.
“We developed our deprescribing guidelines using the same approach that people would use to develop a prescribing guideline,” explains Farrell. The team has also published an instruction manual and methods paper so others can develop further guidelines[11]
.
Using their methods, Farrell estimates that each new deprescribing guideline will take between eight months and one year to develop and will cost between C$80,000 and C$100,000 (not including costs to maintain and update the guideline). “These are the challenges — using a rigorous process like that is very time consuming and expensive,” she says.
“We know [deprescribing] can be done,” says Cheryl Sadowski, a researcher in the Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, who worked with Farrell on the benzodiazepine deprescribing guidelines. “What we’re waiting for from the research in Canada and elsewhere is [whether deprescribing] is always a net benefit for patients.”
There has been an assumption that if harmful medicines are stopped, patients are less likely to experience harm. “But we haven’t proved yet that if we stop, say, your benzodiazepines, you’re going to have fewer falls. Or if we stop your sulphonylurea, you’ll have fewer hypoglycaemic episodes.”
Some research initiatives in Canada, coordinated through CaDeN, are seeking to answer these questions.
CaDeN has called for all treatment guidelines to include recommendations on deprescribing[12]
. This has already happened for dementia guidelines in Canada. For example, clinical practice guidelines for cognitive impairment recommend that the use of atypical antipsychotics by patients with dementia is reassessed periodically, with attempts to taper and discontinue them[13]
. The guidelines also set out when cholinesterase inhibitors should be discontinued – for example, where there is persistent non-adherence, where there are intolerable side effects related to treatment or where comorbidities make continued use of the agent unacceptably risky or futile.
“We hope ten years from now we’re not going to have benzodiazepine deprescribing guidelines, we just hope the insomnia guidelines are written better. That’s our vision,” says Sadowski.

Source: Courtesy of Cheryl Sadowski
Cheryl Sadowski, a researcher in the Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, says that it has not yet been proven whether deprescribing is always a net benefit for patients
Empowered patients
CaDeN has adapted the Bruyère guidelines and produced a suite of freely available resources for prescribers, including deprescribing algorithms, patient handouts and pharmacist-doctor communications.
Its patient handouts are its most widely used resource. They were tested in a randomised trial, known as EMPOWER, with older adults who were chronic users of benzodiazepines.
The handouts, which were distributed by mail via pharmacies, detailed stepwise tapering recommendations and asked participants to discuss these with their doctor or pharmacist, yielded a deprescribing rate of 27% at six months[14]
. “[This result was achieved] even among 94-year-old individuals who had been taking [benzodiazepines] for 40 years,” says Cara Tannenbaum, director of CaDeN. In subsequent interviews, the researchers discovered that two-thirds of handout recipients had taken it to their doctor or pharmacist to discuss discontinuation of their sedative-hypnotic. “But in half these cases, the doctor or pharmacist discouraged the patient from deprescribing,” says Tannenbaum.

Source: Courtesy of Cara Tannenbaum
Cara Tannenbaum, director of the Canadian Deprescribing Network, and her team have developed a tool that pharmacists use to communicate the need for deprescribing to doctors
This work led to the D-Prescribe trial, which sought to educate pharmacists and doctors about the potential risks of certain medicines in older people. The researchers developed a tool that pharmacists used to communicate the need for deprescribing to doctors and sent the EMPOWER brochures to patients. The deprescribing rate was almost 43% at six months[15]
.
CaDeN’s latest focus in on deprescribing tools for opioids that are used to treat chronic, non-cancer pain.
The systematic development and testing of guidelines for deprescribing, spearheaded by Farrell and Tannenbaum in Canada, will go some way to address the barriers around deprescribing and limit the negative impact of polypharmacy on older patients. Patients are open to the idea of deprescribing and will expect practitioners to use evidence-based tools to make appropriate prescribing decisions (see Panel 3).
Reflecting on the response to his deprescribing tweet, Alldred remarks: “There is a real appetite for and a momentum behind [deprescribing]; [for] taking a more sensible approach, instead of piling on medicines to people where there is little evidence.”
Panel 3: Deprescribing tools and resources
NHS Wales: Polypharmacy: guidance for prescribing in frail adults
The Kings Fund: Polypharmacy and medicines optimisation: making it safe and sound
Royal Pharmaceutical Society: Polypharmacy: getting our medicines right. A draft for public consultation
American Geriatrics Society: 2015 updated Beers criteria for potentially inappropriate medication use in older adults
Canadian Deprescribing Network
NHS Scotland: Polypharmacy guidance – medicines review
Best Practice Journal: A practical guide to stopping medicines in older people
BMJ: Using the NO TEARS tool for medication review
NHS England: Stopping over medication of people with a learning disability, autism or both (STOMP) resources
Ontario Pharmacy Evidence Network deprescribing guidelines
NHS North Derbyshire CCG, NHS Erewash CCG, NHS Hardwick CCG, NHS South Derbyshire CCG: Deprescribing: a practical guide
References
[1] Gao L, Maidment I, Matthews FE et al. Medication usage change in older people (65+) in England over 20 years: findings from CFAS I and CFAS II. Age Ageing 2018;47(2):220–225. doi: 10.1093/ageing/afx158
[2] Morgan T, Williamson M, Pirotta M et al. A national census of medicines use: a 24-hour snapshot of Australians aged 50 years and older. Med J Aust 2012;196(1):50–3. doi: 10.5694/mja11.10698
[3] Qato DM, Alexander G, Conti RM et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008;300(24):2867–2878. doi: 10.1001/jama.2008.892
[4] Barnett NL, Oboh L & Smith K. Patient-centred management of polypharmacy: a process for practice. Eur J Hosp Pharm 2016;23(2):113–117. doi: 10.1136/ejhpharm-2015-000762
[5] Reeve E, Wolff JL, Skehan M et al. Assessment of attitudes toward deprescribing in older Medicare beneficiaries in the United States. JAMA Intern Med 2018. doi: 10.1001/jamainternmed.2018.4720
[6] Curran H, Collins R, Fletcher S et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: Effects on cognitive function, sleep, mood and quality of life. Psychol Med 2003;33(7):1223–1237. doi: 10.1017/S0033291703008213
[7] Boghossian TA, Rashid FJ, Thompson W et al. Deprescribing versus continuation of chronic proton pump inhibitor use in adults (Review). Cochrane Database Syst Rev 2017;3:CD011969. doi: 10.1002/14651858.CD011969.pub2
[8] Ballard C, Hanney ML, Theodoulou M et al. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol 2009;8(2):151–157. doi: 10.1016/S1474-4422(08)70295-3
[9] Iyer S, Naganathan V, McLachlan AJ et al. Medication withdrawal trials in people aged 65 years and older. A systematic review. Drugs Aging 2008;25(12):1021–1031. doi: 10.2165/0002512-200825120-00004
[10] Page AT, Clifford RM, Potter K et al. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol 2016;82(3):583–623. doi: 10.1111/bcp.12975
[11] Farrell B, Pottie K, Rojas-Fernandez CH et al. Methodology for developing deprescribing guidelines: using evidence and GRADE to guide recommendations for deprescribing. PLoS One 2016;11(8):e0161248. doi: 10.1371/journal.pone.0161248
[12] Moriarty F, Pottie K, Dolovich L et al. Deprescribing recommendations: an essential consideration for clinical guideline developers. Res Social Adm Pharm 2018. doi: 10.1016/j.sapharm.2018.08.014
[13] Toward Optimized Practice cognitive impairment CPG committee. Cognitive impairment: diagnosis to treatment clinical practice guideline. 2017. Available at: http://www.topalbertadoctors.org (accessed November 2018).
[14] Tannenbaum C, Martin P, Tamblyn R et al. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: The EMPOWER cluster randomized trial. JAMA Intern Med 2014;174(6):890–898. doi: 10.1001/jamainternmed.2014.949
[15] Martin P, Tamblyn R, Benedetti A et al. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA 2018;320(18):1889–1898. doi: 10.1001/jama.2018.16131


