Alamy Stock Photo / MAG
For most people, losing their hair, clump by clump, over the space of a few months is the stuff of nightmares. But for around one or two people per thousand it is reality. People such as 27-year-old Julia, an office administrator from London, who has the autoimmune disease alopecia areata.
“It started when I was around 16,” she says. “My friend noticed a chunk of hair the size of a 5p missing on the back of my head. Before I knew it there were two, then five, then too many to count.” Julia visited a dermatologist but no treatment worked. After six months she’d lost all the hair on her head. Her confidence shot, she didn’t go to university as planned. She still hates social events: “You try to act confident and to love yourself but it’s tough. If I’m honest I’d still do anything to have my hair back.”
Alopecia areata usually strikes before the age of 30. For most people it occurs in transient patches, but for around a quarter of people, whose disease often starts in childhood, it leads to loss of all the hair on their head (alopecia totalis) or entire body (alopecia universalis). Prognosis for hair regrowth is poor, especially with an earlier age of onset and more widespread loss. There are no clinically proven treatments. However, this bleak clinical picture could be about to change as genetic discoveries point the way to new drug targets. But companies with the financial clout to make a difference need to do the necessary clinical trials if new treatments are to be licensed.
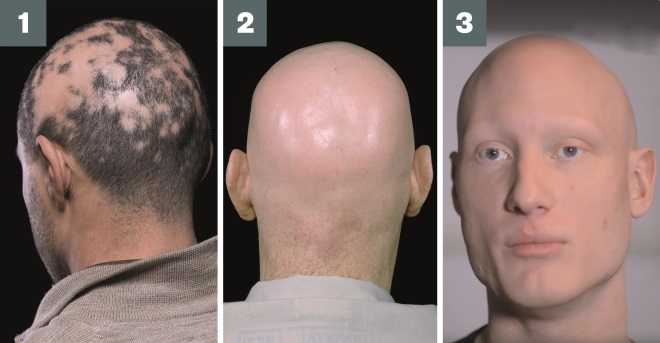
Photo Guide: Alopecia areata
Source: Shutterstock.com / Alamy Stock Photo / Alopecia Universalis: reproduced with permission from HIS Hair Clinic
1) Alopecia areata: loss of hair on the scalp in one or more smooth, round patches
2) Alopecia totalis: loss of all scalp hair
3) Alopecia universalis: loss of all body hair, including eyebrows and eyelashes
“The treatments we have are generally disappointing,” says Ralf Paus, a dermatology researcher who splits his time between the University of Manchester in the UK and the University of Münster in his native Germany. Because it is difficult to differentiate between the effect of a drug and spontaneous regrowth, the true success rate of any treatment is unknown but generally considered to be low. “Especially with advanced or rapidly progressing disease, there’s not really much we can do at the moment before suggesting a wig and hoping for a spontaneous recovery.”
Especially with advanced or rapidly progressing disease, there’s not really much we can do at the moment before suggesting a wig and hoping for a spontaneous recovery
But Paus is optimistic. “We’re getting to a point where we understand more about the disease,” he says. “When we understand more about the disease, we understand why our treatments don’t work and, maybe, we find targets to go after for treatments that do work.”
Hair follicles normally have what is known as immune privilege. Like the brain, testicles, eyes and some other parts of the body, they are protected from the effects of the immune system. However, in alopecia areata, the hair follicles erroneously express autoantigens, danger signals used by cells to tell the immune system to destroy them, usually in the case of infections or cancer.
The immune system obliges. Immune cells swarm the hair follicle and, largely driven by the effects of T cells, prematurely shunt it into a phase of its cycle during which no hair grows. Hair-shaft production and pigmentation are switched off; the damaged hair follicle can no longer hold the old hair shaft, and it falls out or breaks off.
“The main reason that treatments often disappoint is because they don’t get to the base of the problem, the loss of immune privilege,” says Paus. Cortisone treatments, given topically, subdermally or systematically, only suppress the immune system and do so temporarily. “That’s why in some patients you can see regrowth but as soon as the effects of the cortisone wear off, the regrown hairs fall out again.”
The other main treatment option, which Paus describes as “probably the best”, is allergic contact dermatitis. The treatment sensitises a patient to a synthetic allergen, usually diphencyprone, which when increased incrementally shifts the local immune response from the hair-damaging type to a comparatively less-damaging allergic, eczema-like one.
“It works for some patients,” says Paus, “but the chances reduce [in people] with rapidly progressing disease, in young children, and in people on their third or fourth course of the disease.” What we need, he says, is a way to block the process that signals the immune cells in the first place. “You hit that and you restore the immune privilege and hit the disease process right where you need to to bring back the hair regrowth.”
New hope
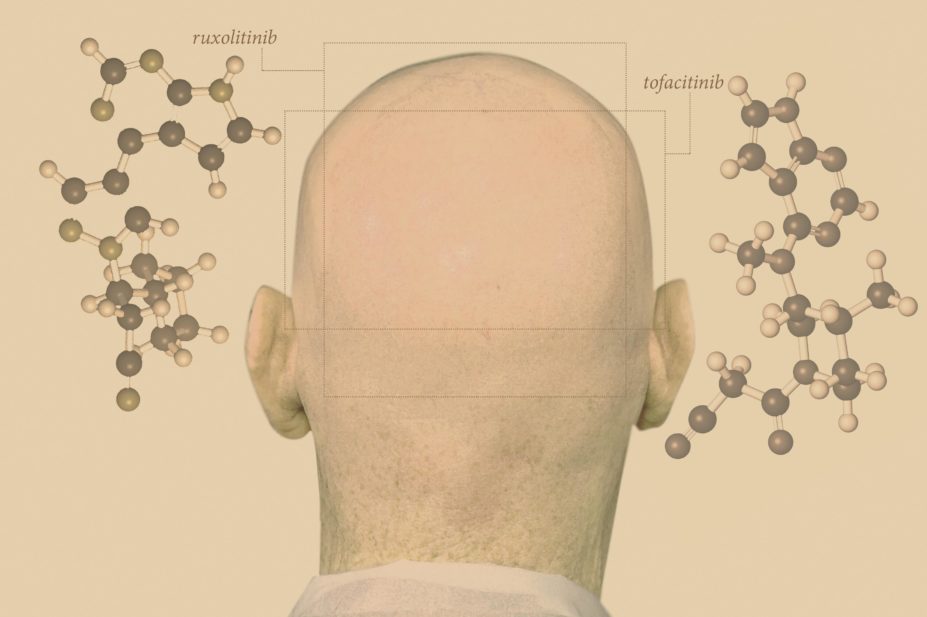
US researchers may have found just such a treatment. In 2014, Angela Christiano, a geneticist from Columbia University, New York, and her colleagues published preliminary findings showing that oral ruxolitinib, a drug approved by the US Food and Drug Administration (FDA) for bone marrow disorder myelofibrosis, restored almost complete hair growth in three individuals with long-standing and severe disease[1]
. Although small, the findings gave hope to the alopecia community almost overnight, an instant impact that belied the number of years that led up to the discovery.
“It was 1996 and I’d just transferred here to Columbia,” says Christiano, who had been studying a rare skin disorder called epidermolysis bullosa, or butterfly disease. “My mentors were encouraging me to find a new research direction and I developed alopecia areata. I have a cousin with universalis so it wasn’t a complete surprise. I just thought ‘OK, alopecia it is’.”
We had to read the genetic tea leaves and let them direct the research
Little was known about the genetics of the disease at that time other than a slight increased risk with a family history of autoimmune disorders. For Christiano, whose hair has since regrown, the way forward was clear — a genome-wide association study (GWAS) to let the genetics of the disease highlight potential mechanisms to target with treatment. “We had to read the genetic tea leaves and let them direct the research,” she says.
By 1999, Christiano and colleagues, with help from the US National Alopecia Areata Foundation (NAAF), had banded together five major clinical research centres and began to interview and phenotype several thousand patients. Their findings, published in 2010, identified eight regions with significant and specific associations with alopecia areata[2]
.
“It opened up an important collaboration for us,” says Christiano, who took the results to an immunologist colleague at Columbia, Raphael Clynes. “We’re just simple geneticists but he looked at the pathways indicated by the GWAS — IL [interleukin]-2, IL-15, IFN [interferon]-y — and said ‘this is just diabetes of the hair follicle’.”
It was an important shift in Christiano’s understanding of what alopecia areata is. “For years, people assumed alopecia was a relative of psoriasis. Most trials just tested existing psoriasis drugs and we wondered why they didn’t work. We never thought about diabetes or coeliac or rheumatoid arthritis but that’s the group of autoimmune diseases we clearly align with.”
In all of these autoimmune diseases a specific autoantigen, or danger signal, is expressed to call in the damaging immune response. “HLA [the human leukocyte antigen system gene complex] was our top hit,” says Christiano of her GWAS findings, “but these are the nuts and bolts of the immune response so we expect them to be upregulated in all autoimmune disorders. What gets geneticists excited are the ones that are unique to a disorder.”
The second biggest hit was for ULBP, a gene unique to alopecia and one, it turned out, that codes for a danger signal expressed by hair follicles in alopecia.
Targeted treatment
When the ULBP-encoded surface protein binds to a killer receptor on the T cell it engages an intracellular cascade that destroys the hair follicle dermal sheath cells and forces hair growth out of its cycle. Several of these intracellular pathways go through tyrosine kinases called janus kinases (JAKs), particularly JAKs 1–3. By inhibiting JAKs 1 and 2, as Christiano and colleagues did with the myelofibrosis drug ruxolitinib, you negate the effect of the danger signal and stop the disease in its tracks.
They’ve tested another JAK inhibitor called tofacitinib, too, which is FDA-approved in the United States for the treatment of rheumatoid arthritis. But as with ruxolitinib, the at-first promising results taper off once the drug is stopped.
It’s not like they lose hair immediately after treatment but maybe we will need to treat for longer and then switch to a topical foam
Christiano and colleagues have tested ruxolitinib and tofacitnib in slightly larger, unpublished studies, and have anecdotal reports from off-label usage in the clinic. “We’re seeing around a 75% response rate,” she says. Such a response is impressive compared with that of currently approved treatments, but unfortunately the effect doesn’t seem to be permanent. The group extended their original ruxolitinib study to 12 patients (not yet published): of the nine who responded, says Christiano, three had significant shedding after stopping treatment. The other six patients reported shedding but it was not as noticeable. “It’s not like they lose hair immediately after treatment but maybe we will need to treat for longer and then switch to a topical foam,” she says. “These things will have to be tested in proper clinical trials.”
One treatment about to enter clinical testing is low-dose IL-2. In a 2014 study, dermatologist Thierry Passeron and colleagues at the University Hospital of Nice, France, reported statistically significant hair regrowth in four of five patients — each of whom had long-standing, treatment-resistant disease — who were given a four-week course of low-dose IL-2 injections[3]
. These results weren’t as celebrated as Christiano and colleagues’ findings because, by Passeron’s own admission, “although two patients had more than 50% regrowth, two had regrowth that wasn’t enough to stop them wearing a wig”.
Two years on and the trial participants, as well as Passeron, are looking back on the trial more favourably. “We have had no relapses,” says Passeron, “even though treatment stopped after the trial.” Not only did the participants not relapse, but their hair continued to regrow: the two women who still had to wear wigs now have almost complete regrowth; of the others, one has almost complete regrowth and the other has more than 75% regrowth. “I’ve never seen such a result — we included very severe patients who had failed up to three types of treatment before and they responded,” he says. But, he warns, there may have been a strong placebo effect since people came to hospital for injections for four weeks.
I’m hopeful in the coming years that the situation for alopecia patients will improve
To test the treatment, with funding from the Programme Hospitalier Recherche Clinique (PHRC), Passeron and colleagues are doing a prospective, placebo-controlled randomised trial in 80 patients around France and following them up for one year. They will take blood samples to test a hypotheses supported by biopsies from the five patients in the 2014 open-label study: that the low-dose IL-2 stimulates the activity of T regulator cells that dampen down the damaging T effector cells and reduce the immune infiltrate around the hair follicle. “If our hypothesis is true, and the result is good,” says Passeron, “then, with the impressive JAK inhibitors, I’m hopeful in the coming years that the situation for alopecia patients will improve.”
Out of gear with industry
Paus says there are several other promising treatments that could be taken forward for testing, though a seeming disinterest from pharmaceutical companies means most of these remain on laboratory shelves. It’s concerning, he says, given that a range of drugs that target different mechanisms in different individuals are likely to be needed. At the 2015 World Congress for Hair Research in Miami, Florida, Paus proposed that alopecia areata doesn’t actually exist as a disease entity. “I think alopecia areata is just a stereotypical response of the hair follicles to different types of inflammatory damage when immune privilege collapses. If this hypothesis [that there might be sub-categories of alopecia areata caused by different mechanisms] is true, we will need a personalised medicine approach.”
Christiano echoes Paus’s suggestion, saying that the existence of subtypes of disease could explain why roughly a quarter of patients don’t seem to respond to JAK inhibitors. “The pharmaceutical industry needs to do the registration trials on the JAK inhibitors,” she says, “that way insurance companies will cover it so people don’t have to pay out of pocket. It would also pave the way for future trials in children, which is far and away the overwhelming demand right now.”
Prescribing JAK inhibitors off-label to adults is one thing, says Christiano, but because side effects include an increased risk of infections or cancer, giving them to children without a proper understanding of safety is several steps removed. Use of topical formulations would lessen this risk.
Incyte Pharmaceuticals, based in Alapocas, Delaware, has begun testing topical ruxolitinib for alopecia, says Christiano. Meanwhile Pfizer, which owns tofacitinib, has expressed little interest in alopecia areata as an indication, instead focusing on psoriasis for which there is a better known and thus more reliable market.
Our experience is that the larger, more experienced drug companies haven’t been eager to donate drugs or fund trials for alopecia areata as a new indication
“Our experience is that the larger, more experienced drug companies haven’t been eager to donate drugs or fund trials for alopecia areata as a new indication,” says Dory Kranz, president and chief executive officer of NAAF. “We’re seeing more interest from the smaller, more nimble companies that are developing their own molecules or acquiring molecules that they’ll bring to market quickly.”
NAAF is currently helping six companies develop new products. Since its inception in 1981, NAAF has awarded US$4.4m in grants to further alopecia research, but it’s aware that at an average cost of US$1bn over 15 years, it will need to engage the big guns of the biopharmaceutical industry to bring promising new treatments to market.
“We also need to show to these companies that there will be a willingness to pay for these treatments to lessen the element of risk,” says Kranz.
Paus believes there is another reason why researchers and drug companies should invest in alopecia areata research: a unique opportunity to study a model autoimmune disease that can teach what goes wrong when the immune system attacks previously healthy tissue, as it does in diseases such as multiple sclerosis, diabetes and autoimmune uveitis.
“You’ve only got one brain, one pancreas and two eyes — how easily are you going to cut them out and do research to learn all the tricks that the body uses to evade being attacked by the immune system?” he asks. “Well you have five million hair follicles you can play with so you better start using the follicle as a study model.”
It sounds funny, but feeling that you’re not being helped is somehow an improvement on feeling helpless, which we’ve all felt for so long
Until this research gains traction with industry, not much will change for people like Julia. “Knowing that there might be a possibility of treatment helps in a way,” she says. “It sounds funny, but feeling that you’re not being helped is somehow an improvement on feeling helpless, which we’ve all felt for so long.”
There is definitely a will from people with alopecia areata to find a treatment, the question that NAAF and others will be asking is how they find the way, a prospect that Paus is upbeat about. “It’s frustrating that work on more effective treatments for alopecia areata is not benefitting our patients faster,” he says. “But we should remember two things: never before has work on this progressed as fast as it has done in the past few years, and even when no treatment has worked, the patient’s hair follicle will just hang in there and will be ready to respond once that long-sought therapy finally becomes available.”
- This article was amended on 12 May 2016 to clarify that NAAF was founded in 1981, not 1985 as originally stated.
References
[1] Xing L, Dai Z, Jabbari A et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nature Medicine 2014;20:1043–1049. doi: 10.1038/nm.3645
[2] Petukhova L, Duvic M, Hordinsky M et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010;466:113–117. doi: 10.1038/nature09114
[3] Castela E, Le Duff F, Butori C et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatology 2014;150:748–751. doi: 10.1001/jamadermatol.2014.504