Paul Stuart
In its ten-year health plan for England, published in July 2025, the UK government pledged to put the NHS “at the front of the global genomics revolution”, teasing a “genomics population health service to provide predictive and preventative care that anticipates need, rather than just reacting to it”.
With plans to roll out universal newborn whole genome sequencing, a national dataset of patients and health information, pharmacogenomics as part of the pharmacy undergraduate curriculum, and mandatory genomic tests already commissioned for some drugs, the UK certainly has potential to expand its use of pharmacogenomics to improve care for patients.
However, a lack of joined-up systems and the absence of a fully-costed implementation strategy for pharmacogenomic-based prescribing has led to slow progress in expanding access to tests, improving affordability for the health system and preparing the workforce.
And while paediatrics is leading the way in genomic testing for rare diseases, the economic case for large-scale pharmacogenomic prescribing in the generally healthy paediatric population is yet to be established.
These were the overarching messages from a roundtable meeting, hosted by The Pharmaceutical Journal at the Royal Pharmaceutical Society (RPS) London headquarters on 9 October 2025.
The roundtable, supported by BNF Partnership as part of BNFC‘s 20th anniversary, brought together a range of experts to discuss the challenges and opportunities around wider implementation of paediatric pharmacogenomics (see Box).
The event was co-chaired by Alex Clabburn and Katherine Sole, research and learning editors at The Pharmaceutical Journal.
Box 1: Roundtable attendees
- Charlotte Barker, NIHR advanced fellow, UCL Great Ormond Street Institute of Child Health and honourary research fellow, University College London;
- Neil Caldwell, consultant pharmacist, children’s services, Wirral University Teaching Hospital, deputy chair of the paediatric formulary committee for BNFC;
- Jelena Feenstra, senior manager in medical affairs, Thermo Fisher Scientific;
- Richard Hastings, consultant paediatrician at Sherwood Forest Hospitals NHS Foundation Trust;
- Daniel Hawcutt, professor of paediatric clinical pharmacology, University of Liverpool;
- Nicola Husain, education and training lead, South East Genomic Medicine Service;
- Dhavendra Kumar, honorary clinical professor, clinical pharmacology and precision medicine, Queen Mary University, and honorary consultant clinical geneticist, St. Bart’s Hospital, The Genomic Medicine Foundation UK;
- Jackie O’Brien, director, the UK Industry Pharmacogenetic Network;
- Munir Pirmohamed, David Weatherall chair in medicine at the University of Liverpool, NHS chair of pharmacogenetics, and a consultant physician at the Royal Liverpool University Hospital;
- Hayley Wickens, consultant pharmacist genomic medicine, South and Central Genomics.
Opportunities and challenges
As the “doorway for rare diseases”, Daniel Hawcutt, professor of paediatric clinical pharmacology at the University of Liverpool, explained that paediatric teams are used to dealing with complex and rare conditions, genetic testing, gathering consent and working through ethical considerations such as DNA cascade testing within the wider family unit.

Paul Stuart
We’ve got small numbers of patients, no joined-up thinking, a lot of gaps in the evidence and some challenges
Daniel Hawcutt, professor of paediatric clinical pharmacology at the University of Liverpool
In children, genomic testing is performed where symptoms suggest an inherited disease or where family risk factors or the newborn spot test suggest a need for further genomic testing. It is also used in paediatric and adult care to guide prescribing for drugs where toxicity owing to a genetic difference presents a risk for patient safety.
However, existing variability in pathways would be difficult to scale across the whole health system, Hawcutt suggested.
“We’ve got small numbers of patients, no joined-up thinking, a lot of gaps in the evidence and some challenges,” he said.
Paediatrics also poses its own unique challenges, preventing simple translation of adult pharmacogenomics to children, Hawcutt explained (see Figure 1). This includes different diseases, physiology, polypharmacy and DNA expression, leading to different risk scores for tests, different uses for drugs and therefore different personalised therapy for children.
“It will be different from adult personalised therapy, and it needs input from paediatricians to guide it,” Hawcutt said.
Additional considerations for pharmacogenomic prescribing in children that were highlighted by attendees are summarised in Figure 1.
Figure 1: Additional considerations for pharmacogenomic prescribing in children compared with adults
Scaling pharmacogenomics
The NHS has two main options for developing pharmacogenomics in the future: single or panel-based testing — looking for specific known DNA variants that have a pharmacogenomic application — or to sequence the entire genome and use this as a basis for pharmacogenomic prescribing as more becomes known about different variants in the future.
Currently, there is a lack of clarity about how pharmacogenomics will be used in the NHS. Implementation studies — such as the ‘Pharmacogenetics Roll Out – Gauging Response to Service’ (PROGRESS) trial — are seeking to establish the feasibility of NHS-wide diagnostic services. However, without firm commitments and detailed plans, the diagnostics and device industry is unable to develop the technologies and services that the NHS needs or unlock the cost reductions that come with large-scale use.
The cost of the testing always will depend on what the guidelines will say, and how many markers in which population will be tested for
Jelena Feenstra, senior manager of medical affairs at Thermo Fisher Scientific
“The cost of the testing always will depend on what the guidelines will say, and how many markers in which population will be tested for,” Jelena Feenstra, senior manager of medical affairs at Thermo Fisher Scientific, told attendees.
“As industry, we want to know, what are the expert views of how overall [pharmacogenomics] will develop over the next three to five years, because we are keen to develop technology,” she added.

Paul Stuart
At the roundtable, Jackie O’Brien, director of the UK Industry Pharmacogenetic Network, set out the steps required for industry to effectively partner with the NHS to support genomic testing, summarised in Figure 2.
Figure 2: What steps are needed for industry to partner with the NHS on genomic testing?
Panel or single indicator testing
Single or panel indicator testing is currently used in the NHS and is therefore expected to be the basis for large-scale roll out. Currently, hundreds of genomic tests are available to NHS clinicians to test for specific conditions and prescribing considerations, through national genomic test directories.
Hayley Wickens, consultant pharmacist genomic medicine, South and Central Genomics, explained that this means a “mixed model” of different test types, panels and turnaround times is needed from industry.
New tests can be developed and added to the directory. At the roundtable, Munir Pirmohamed, David Weatherall chair in medicine at the University of Liverpool, NHS chair of pharmacogenetics, and a consultant physician at the Royal Liverpool University Hospital, said he was open to working with others on developing areas that need a guideline.

Paul Stuart
Pirmohamed leads the Centre for Excellence in Regulatory Science and Innovation (CERSI) in Pharmacogenomics, which was set up to provide clarity to clinicians and industry on how pharmacogenomics can be used, create clinical pathways that include pharmacogenomic prescribing, as well as regulatory pathways to facilitate innovation and implementation.
The group takes a “whole life course” approach, developing guidelines for use in both adult and paediatric patients, with input from both adult and paediatric clinicians, Pirmohamed explained.
However, funding for the centre only extends to March 2026, while money for implementing the guidelines in practice is lacking.
NHS England’s ‘Medium-term planning framework’, published in October 2025, told ICBs that providers are expected to deliver services in line with the NHS Genomic Medicine Service specification from April 2026, including the delivery of genomic testing services and testing strategies as well as clinical functions for cancer, rare disease and population health and the new genomics population health service.
But Richard Hastings, consultant paediatrician at Sherwood Forest Hospitals NHS Foundation Trust, said: “Even if we were told that we have to do that test… there’s no money to do it”.
Neil Caldwell, consultant pharmacist in children’s services at Wirral University Teaching Hospital, told attendees that there was “no money” for gentamicin testing for newborns in district general hospitals in England.
“Yes, the test is available; yes, it will benefit one in 400 babies… but in this present climate, there is no money… With the best will in the world, it’s not going to happen,” he said.
This localised approach contrasts with Scotland, whose government has funded a national roll-out of the test.

Paul Stuart
Joined-up systems are needed
While panel testing is the method currently used, “we are moving, over the long term, to genome sequencing, and eventually everybody will have a genome sequence… That’s the direction of travel,” Pirmohamed explained.
“There’s more whole genomes done in this country than any other country. But at the moment, the pharmacogenomics data [are] not available,” he added. Figure 3 provides an overview of the current landscape.
Pharmacogenomic information — whether from panel testing, exome sequencing or the eventual whole genome sequencing — needs to be stored somewhere accessible for potential use in prescribing throughout the patient’s lifetime.
Figure 3: The current landscape of genomic testing in the UK
Information storage
“If we’re going to start genotyping newborns, there’s going to be a tsunami of incidental genomes found,” Hawcutt suggested.
But adding any pharmacogenomic information to a patient’s record can be complicated, particularly for children. Roundtable attendees discussed practicalities and questions around consent (see Figure 4).
Figure 4: Specific considerations for children
Since information is currently limited, workarounds are used — patients sharing their own test results with prescribers, who may be unused to making pharmacogenomic prescribing decisions — and ‘allergy’ sections of electronic patient care record (EPR) are used to flag pharmacogenomic data.
However, roundtable attendees debated whether the information in this section — particularly if not immediately actionable or relevant to every clinician — might lead to information fatigue. And others noted that pharmacogenomic information could be built into clinical systems, but healthcare providers may not pay for or use them.
In other instances, genomic data are collected but not stored anywhere clinicians can access. For instance, in Scotland, under the Phoenix trial, pharmacogenomic data are processed only for the purposes of the trial and not added to patient records.
Roundtable attendees also discussed the role of the NHS App, which is intended to bring together patient access to information held by NHS clinical systems. But as well as the infrastructure needed to appropriately store genomic information, the roundtable concluded that consideration will need to be given to what access patients, parents and carers should have to this information (see Figure 4) — particularly since patients aged under 18 years cannot access the NHS App and that genetics information may have consequences for wider family members.
Training the workforce
At present, utilisation of pharmacogenomic testing is limited, so can be managed by clinical pharmacologists and clinicians within the few specialties using it.
However, as pharmacogenomic prescribing becomes more widespread, Caldwell explained that both the wider healthcare team and the general public need to understand “that there’s different factors that could affect you or your family’s response to treatment”.
You have to take the pharmacogenomic result into consideration of everything else about the patients and make a holistic decision
Nicola Husain, education and training lead, South East Genomic Medicine Service
“But different clinicians within that need more developed [training]”, that could be added as and when it was needed, he suggested.
Basic understanding needed by all
“Anybody can read a table and say, ‘oh, they’re this, so do this,’” said Nicola Husain, education and training lead at the South East Genomic Medicine Service. But there’s a “big need” for prescribing clinicians to “understand the underpinnings of the pharmacogenomic testing in conjunction with everything else that informs prescribing”, she suggested.
“You have to take the pharmacogenomic result into consideration of everything else about the patients and make a holistic decision,” Husain added.

Paul Stuart
Under the General Pharmaceutical Council (GPhC)’s 2021 ‘Initial education and training standards for pharmacists’, to pass the MPharm degree from 2025, students must show how they would apply clinical therapeutics, pharmacology and genomic principles to make effective use of medicines for people, including within prescribing practice. And they must demonstrate this within their foundation training year.
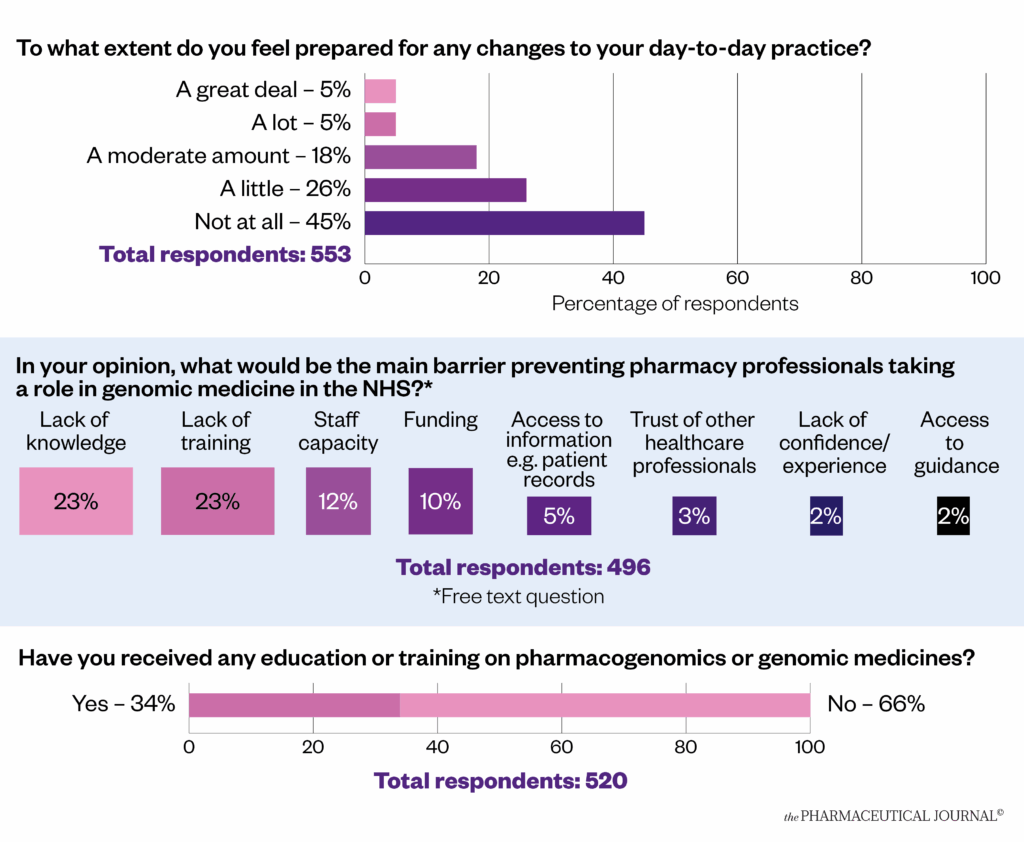
However, results of a 2022 survey carried out by The Pharmaceutical Journal suggested a need to train the existing workforce (see Figure 5). Almost half (46%) of more than 600 pharmacy professional respondents (n=496) said a lack of knowledge and training would prevent pharmacy professionals getting involved in genomic medicine. Two-thirds of respondents (66%; n=520) said they had never received any education on pharmacogenomics, while almost half (45%; n=553) said they felt “not at all” prepared for the changes to day-to-day practice it would bring.
Figure 5: The Pharmaceutical Journal reader survey on pharmacogenomics preparedness (2022)
The Pharmaceutical Journal
‘Just in time’ resources to support pharmacogenomic prescribing
Even once trained, without regular and immediate use of pharmacogenomic prescribing via a commissioned NHS service, it will be difficult for pharmacists to maintain their skillset, both Wickens and Pirmohamed noted.
Instead, Wickens suggested that “just-in-time resources” could be used to upskill clinicians as and when specific tests and prescribing pathways come into practice. Further considerations on workforce training needs discussed by attendees are summarised in Figure 6, with existing education resources listed in Box 2.
Figure 6: Comments around training the workforce

The Pharmaceutical Journal
Box 2: Pharmacogenomics education resources
- UK Clinical Pharmacy Association: a basic understanding of pharmacogenomics for pharmacy teams and further resources for a more developed understanding;
- Advanced genomics knowledge guide for pharmacists, UK Clinical Pharmacy Association;
- GeNotes, part of the Genomic Education Programme, Health Education England;
- The Specialist Pharmacy Service also recommends resources to help healthcare professionals answer questions about pharmacogenomics.
Decision support systems
Pirmohamed suggested that decision support systems — registered as a class 2a medical device — could also be used. Hawcutt stressed that any education or resource developed must include information about children from the outset, rather than as an afterthought.
O’Brien suggested to the group that industry could play a role in developing training resources to sit alongside NHS pathways as a direction of travel for pharmacogenomics use in the NHS became clear.

Paul Stuart
Industry is required to provide educational materials as part of what we do… if you are creating things, it would be great to be part of the conversation
Jackie O’Brien, director of the UK Industry Pharmacogenetic Network
“Industry is required to provide educational materials as part of what we do… if you are creating things, it would be great to be part of the conversation,” she told other roundtable attendees.
However, she did stress that industry would not be able to fund any resource that incorporated off-label prescribing, which is commonly used in paediatric patients.
Meanwhile, Pirmohamed warned that devices developed in parallel — such as ProgressRx in North West England and a system built on the Scottish drug interaction support system — should be designed to work with each other and with existing systems.
“People are just doing exactly what we did with electronic health records in this country in secondary care… everybody develops their own homegrown systems, and they don’t talk to each other… we should be learning lessons from that,” he said.
Genomics ‘champions’ to support colleagues
Hawcutt suggested that more clinicians would need to be trained as clinical genomic specialists, perhaps by using special interest (SPIN) modules, and then act as genomics ‘champions’ within their departments.
“You can’t have four pharmacologists in an ivory tower running this — it’s got to be a diaspora of people who have a level of knowledge to manage the paediatric situation,” he said.
Pirmohamad suggested that around 15% of clinicians might take on a more specialised role in pharmacogenomics — a similar proportion to other specialties such as cardiovascular or diabetes pharmacists. “I think that’s a good target,” he said.
Pharmacogenomic specialists available on demand?
Complex cases involving children usually involve a general paediatrician as a clinical lead, who would then work with the different specialties, but attendees’ opinions about whether paediatricians would want to specialise in genomics were mixed.
Charlotte Barker, specialist registrar clinical genetics at Guy’s and St Thomas’ NHS Foundation Trust, suggested that pharmacogenomic experts could be available to contact via video consultations.
“Because of the normality now of virtual consultations, it might be that you could have an expert who are only based at a small number of centres, but who could still provide input through a virtual consultation for children or their families in regions far away geographically.”
She suggested that this could work well in paediatrics, where pharmacogenomics would likely begin to be rolled out in tertiary and secondary care, given this is where the burden of illness and therefore use of medication in children is generally concentrated around rare diseases and cancer.
“In clinical genetics, for some of our ultra rare diseases, it’s quite normal to have a highly specialised commissioned service… where patients, sometimes from all over the UK, will come to one specialist centre,” she added.

Paul Stuart
Conclusion
Pharmacogenomic prescribing looks likely to continue its gradual roll out with new use-cases and pathways implemented incrementally, beginning with the most cost-effective applications, as per NHS England’s genomic strategy with approaches to adoption and service design continuing to vary geographically.
However, joined-up systems for information sharing and consent are not yet developed and, without clarity on long-term resourcing requirements, the UK could miss its opportunity to assume a world-leading position on pharmacogenomic prescribing. Information collected now, including exome and genome sequencing, should be future-proofed — with appropriate consent, storage and training for clinicians — so that it can be used in prescribing when current paediatric patients present with further health conditions later in life.
Roundtable attendees also stressed that new systems currently in development in different parts of the UK should be integrated. The current decision-making and approval timeframe for new testing applications is currently not aligned with the government strategy of creating a genomics-based health system by 2035. With limited NHS budgets and an overstretched workforce, efficient collaboration with industry will be needed. But this requires a long-term commitment from the NHS, backed up with front-loaded funding that can support the creation of the infrastructure and workforce that will be required, rather than a piece-meal approach based on short-term funding decisions and isolated use-cases.

Paul Stuart

BNF Partnership comprises BMJ Group, Pharmaceutical Press, Royal College of Paediatrics and Child Health, and Neonatal & Paediatric Pharmacy Group.