Mclean
Out of the tragedy of the COVID-19 pandemic, a glimmer of something remarkable appeared — a vaccine technology that worked like no other was pushed firmly into the spotlight. And it delivered. The messenger RNA (mRNA)-based COVID-19 vaccines, developed by BioNTech and Moderna in under a year, changed the course of the pandemic.
However, the technology that allowed these vaccines to make it into people’s arms at record speed did not arise out of thin air: it has been slowly progressing for more than 20 years. Before COVID-19 forced mRNA vaccines onto the global stage, researchers were diligently working with mRNA to find a therapeutic vaccine for cancer. And, with this new-found pandemic fame, came a renewed vigour for the whole field.
“The COVID-19 pandemic has certainly led to an unprecedented increase of investment into this technology,” says Ulrike Gnad-Vogt, senior vice president and area head (oncology) at CureVac, an mRNA biotech company based in Tübingen, Germany.
“This has not only validated the approach scientifically but enabled significant growth in the field due to availability of funding, which was just not there before.”
Early work
mRNA vaccines work by carrying instructions that tell the body’s cells to make particular proteins. These proteins are then recognised by the immune system as foreign, and antibodies are produced. Once the proteins are made, the mRNA has done its job and degrades. If this degradation did not happen, then proteins would be produced in perpetuity. This fragility is the reason why, initially, mRNA was seen as too difficult to work with.
Despite this perceived instability, using mRNA remained a tantalising prospect for some cancer vaccine researchers. Kim Lyerly, professor of surgery, pathology and immunology, was working in the late 1990s at Duke University, Durham, North Carolina, where he collaborated with fellow faculty and cancer immunologist Eli Gilboa, making vaccines with dendritic cells, which show antigens to the surface of T-cells to train the immune system to recognise and destroy them.
The researchers took genetically identical mice with identical tumours and immunised the mice using mRNA in dendritic cells. They saw that the mice’s immune system became educated against the tumour, ultimately destroying it[1]. The crucial step, Lyerly says, was getting the mRNA into the dendritic cells early, so that it remained stable.
Naked mRNA is very often degraded rapidly by ribonoclease enzymes. Getting it into cells allows it be translated into proteins, which are much more stable
Kim Lyerly, professor of Surgery, Pathology and Immunology, Duke University
“Naked mRNA is very often degraded rapidly by ribonoclease (RNase) enzymes. Getting it into cells allows it be translated into proteins, which are much more stable,” explains Lyerly.
This proof of concept was vital in the story of mRNA cancer vaccines.
Around the same time, researchers at Vrije Universiteit Brussel (VUB) were also looking at dendritic cells as part of cancer vaccines. This work led to the development of an mRNA-based adjuvant whereby, in addition to tumour-associated antigens, dendritic cells contain three mRNAs that encode for three immune-stimulating proteins[2]. Once in the body, these dendritic cells are able to migrate to the lymph nodes and activate the T-cell response.
With this body of research growing stronger and broader, commercialisation was an obvious next step.
Commercialisation
CureVac, founded in 2000, was the first company to develop and run clinical studies with mRNA vaccine technology, initially targeting cancer but moving on to other disease areas (see Box).
Then, in 2008, BioNTech, based in Mainz, Germany, was founded with the specific aim of developing personalised cancer therapies based on mRNA. Following this was Moderna, founded in 2010, and several other mRNA start-ups, including eTheRNA in Belgium, which was borne from the work at VUB.
Unlike vaccines for cervical cancer, where a virus underlies the disease, mRNA vaccines are used as a therapy, teaching the body how to fight the cancer once it is already present.
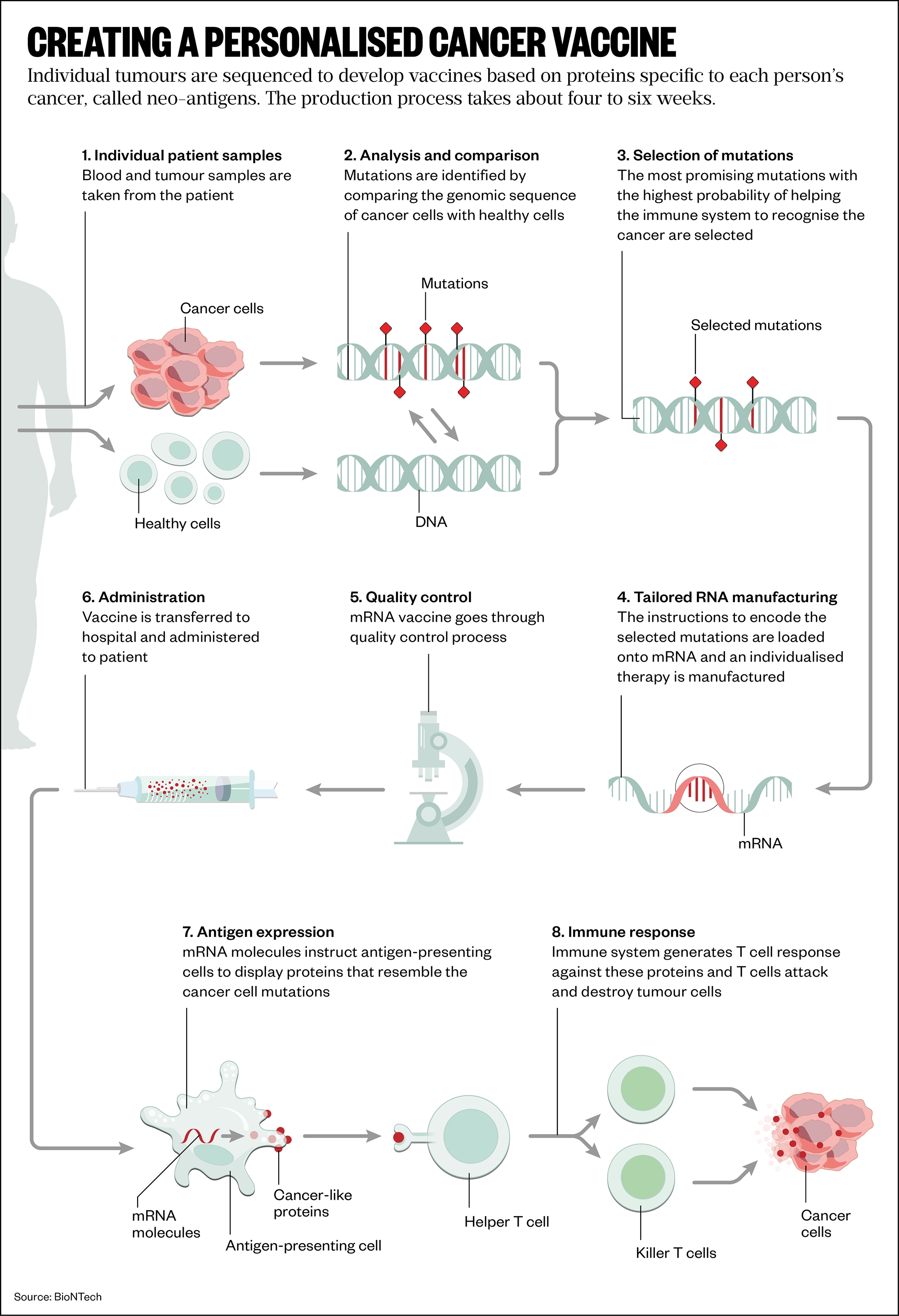
There are two main strategies being used to develop mRNA cancer vaccines. First, a generalised vaccine whereby researchers find proteins that are common to a group of cancers and develop mRNA that can make those proteins, similar to the work at VUB. Second, a highly personalised vaccine whereby individual tumours are sequenced to develop vaccines based on proteins specific to each person’s cancer, called neo-antigens.
Some progress is being made in making a generalised cancer vaccine, which is attractive because it is cheaper than going down the personalised medicine route. For example, Karine Breckpot, head of the Laboratory for Molecular and Cellular Therapy at VUB, has shown in various mouse cancer models that the mRNA-based adjuvant can render tumours into an autovaccine without further addition of antigen mRNA[3]. This approach is “appealing”, she says, because it removes the need to search for the best target neo-antigens (see Figure).
Nevertheless, personalised vaccine technology is further ahead for now.
For any of the mRNA vaccines to work, the mRNA needs to be combined into a deliverable format and the most common way to do that is to encapsulate it in a lipid nanoparticle. At low pH, these tiny particles carry a positive charge, allowing genetic material to form a complex inside the lipid shell. Once in the body, the pH changes and the particles become neutral, allowing them to deliver their cargo safely.
Box: mRNA vaccines for other diseases
Cancer is not the only disease that will benefit from new investment in mRNA vaccine technology.
Vaccines based on mRNA are also being developed for a range of viruses. CureVac is working on vaccines against Lassa and yellow fever, influenza and rabies, while Moderna is developing vaccines against CMV (cytomegalovirus), Epstein-Barr virus, herpes, HIV and public health-related Zika and Nipah viruses.
COVID-19 has shown the world how flexible and rapidly deployed mRNA technology can be. This will provide a push for those developing other vaccines against highly infectious diseases. The spike protein that was targeted in the COVID-19 vaccines is also a key target for vaccines against other deadly coronaviruses, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS).
Tandem attack
Once the mRNA vaccines have successfully made it into the body, they can be used in tandem with other therapies to trigger a strong immune response, the most promising of which are immune checkpoint inhibitors[4]. Checkpoint proteins can stop the body’s immune system from responding to cancer cells, so inactivating these with checkpoint inhibitors allows the immune system to continue to fight the tumour.
“The idea that we can create vaccines that trigger immune responses against cancer and that we also know how to make sure that the immune system doesn’t shut it down, that’s where there’s so much promise now for the combination of vaccines plus immune checkpoint,” explains Lyerly.
This combination of therapies is essential in the fight against ever-moving targets, like mutating cancer cells, agrees CureVac’s Gnad-Vogt.
Combination with other treatment modalities, like checkpoint inhibitors, is key, at least in patients with advanced cancer, but this makes the clinical development also more complex
Ulrike Gnad-Vogt, senior vice president and area head (oncology), CureVac
“Cancers have developed mechanisms to escape from immune attack and, even if a cancer vaccine induces immune responses, there is no guarantee it is sufficient to control tumour growth. Combination with other treatment modalities, like checkpoint inhibitors, is key, at least in patients with advanced cancer, but this makes the clinical development also more complex.”
And, even when the vaccines use personalised neo-antigens, results have been mixed, says Breckpot. “For certain patients, its beneficial, for other patients it doesn’t do anything,” she says. “We need a better understanding of why certain patients benefit from this type of vaccine while others don’t.”
For example, in 2020, Roche and BioNTech reported a response rate of just 8% in a phase Ib trial of their neo-antigen vaccine RO7198457 in combination with monoclonal antibody atezolizumab against solid tumours[5]. However, more promising news came in July 2022, when French company Transgene announced positive results from a small-scale phase I trial of its neo-antigen vaccine TG4050. Four patients with ovarian or head and neck cancers showed advanced T-cell activity after a nine-week treatment with the vaccine[6].
Some researchers are even working to develop mRNA vaccines that will overcome resistance to cancer medicines. Breast cancer, for example, is often treated with anti-oestrogen therapy tamoxifen. However, tamoxifen has a limited lifetime because eventually the cancer will mutate and become resistant to it. Lyerly’s team is now developing a vaccine that targets that mutation. He says the team has unpublished animal data that suggest the approach works.
“The principle can be applied to other cancers that eventually become resistant to therapies,” Lyerly says. “Every therapy that fails, we have a solution for it based on these vaccines.”
Manufacturing
With mRNA established as a workable technology, attention has turned to the vaccine manufacturing process. A huge advantage of mRNA vaccines is that they are far easier and quicker to make than conventional vaccines, which have historically taken 10–15 years to develop and use disabled forms of bacteria, viruses or protein molecules.
These conventional vaccines are manufactured in cell cultures and require large factories, whereas the manufacturing process for mRNA vaccines is cell-free and avoids the need to grow highly pathogenic organisms on a large scale, reducing the risks of contamination or release of dangerous pathogens.
The way mRNA is made, is a much simpler process compared to how conventional vaccines are made
Zoltan Kis, expert in mRNA vaccine production, University of Sheffield
Zoltan Kis, an expert in mRNA vaccine production at the University of Sheffield, explains why this is important: “You don’t have to make the actual antigens you will only make the mRNA. And the way mRNA is made, is a much simpler process compared to how conventional vaccines are made.”
Because antigens are not needed, the process does not require living cells, he adds, just a synthetic reaction involving enzymes. Coupled with the advances in lipid nanotechnology made over the years, and infrastructure developed rapidly during the pandemic, a new vaccine could be ready to trial in as little as two weeks, Kis suggests.
In principle, he adds, once the infrastructure is in place, you can make any kind of mRNA, you can make any kind of cancer protein and, in turn, any kind of cancer therapy.
The pipeline
Given the speed with which the COVID-19 vaccines made it into clinics, why have cancer vaccines stalled?
“Unlike SARS-CoV-2, which affects humans similarly, cancer in each individual is unique, necessitating a more personalised approach,” explains Praveen Aanur, vice president and therapeutic area head (oncology) at Moderna.
This means that each tumour must be sequenced to identify the relevant peptides before the mRNA can be made.
The development of personalised mRNA-based therapeutics to treat diseases like cancer is a frontier that is only at the beginning of its potential
Praveen Aanur, vice president and therapeutic area head (oncology), Moderna
“The development of personalised mRNA-based therapeutics to treat diseases like cancer is a frontier that is only at the beginning of its potential,” says Aanur, but the work on COVID-19 vaccines will benefit the development of cancer vaccines, he adds.
“All the information that came out of the vaccines with COVID-19 [will] help us, guide us in the right direction for the nanoparticle we use, the dendritic cells, to really have a good vaccine,” Breckpot adds.
Moderna, CureVac and BioNTech have several cancer vaccines in their pipelines in association with various pharmaceutical companies and positive results are starting to filter through (see Table).
In June 2022, BioNTech reported positive phase I trial data for a pancreatic cancer vaccine being developed in partnership with Genentech[7]. The vaccine is based on an individualised neo-antigen coupled with a checkpoint inhibitor. In the phase I trial, 16 patients were vaccinated with BioNTech’s mRNA-based individualised neo-antigen specific immunotherapy (iNeST) autogene cevumeran, as well as the anti-PD-L1 immune checkpoint inhibitor atezolizumab, alongside chemotherapy. In 8 of the 16 patients dosed, they remained cancer-free 18 months after being treated.
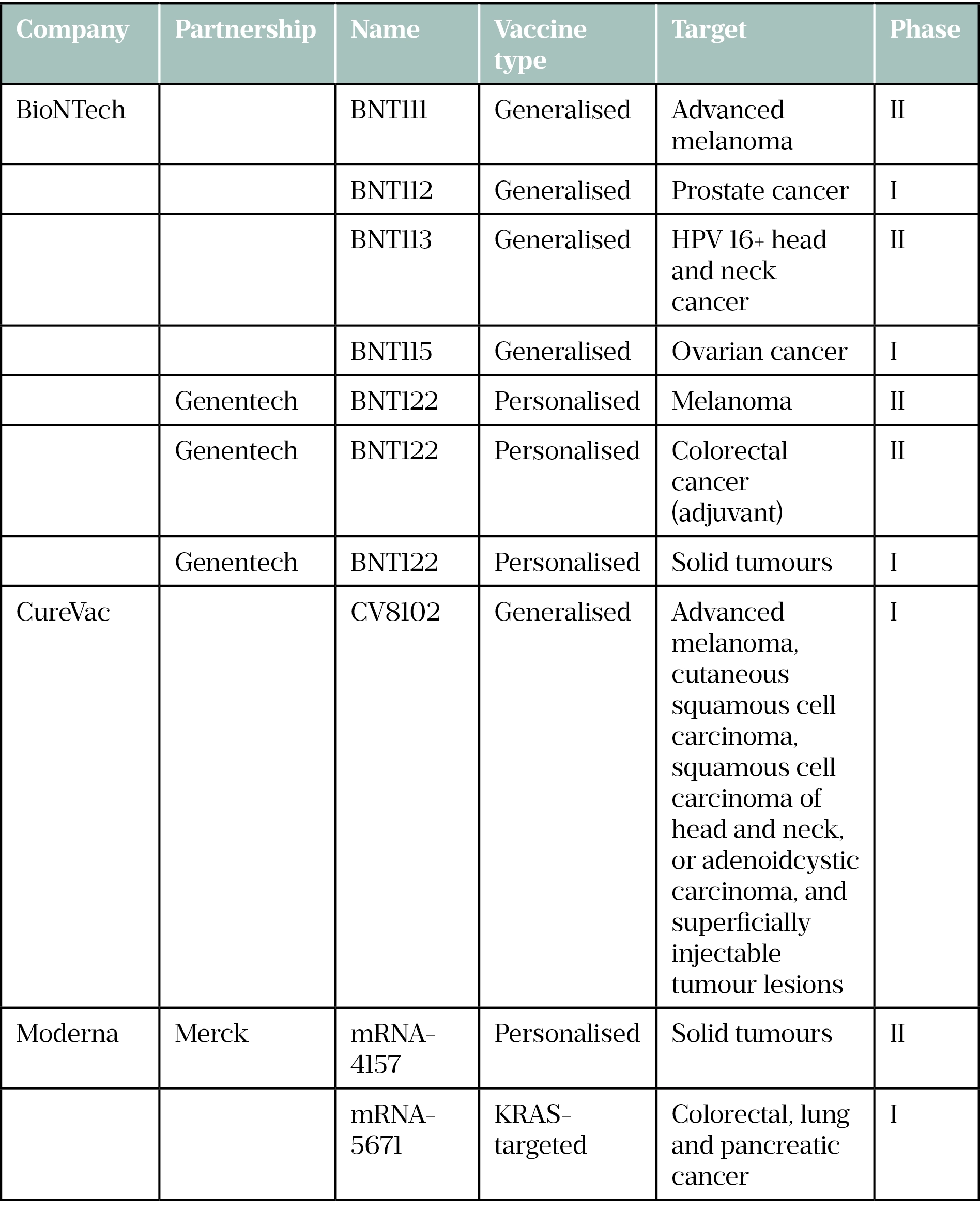
KRAS: Kirsten rat sarcoma viral oncogene homolog
Trial evolution
Another consequence of the COVID-19 pandemic that could benefit the development of mRNA cancer vaccines is the speed that the COVID-19 vaccines whipped through clinical trials.
I think the way that we do clinical trials has been changed forever
Sam Godfrey, research information team leader, Cancer Research UK
“I think the way that we do clinical trials has been changed forever,” says Sam Godfrey, research information team leader at Cancer Research UK.
“COVID-19, in particular, led to some of the fastest clinical process progress we’ve ever seen,” Godfrey says. “Now we’re going to see smaller trials, faster, more agile trials, I think, which is great, because it also brings the cost down.”
And, as a signal of the increased investability of mRNA cancer vaccine technology, in May 2022, CureVac announced a partnership with bioinformatics-based immunotherapy business, myNeo, to help speed up its antigen-identifying process. The following month, CureVac acquired Frame cancer therapeutics, a company that specialises in antigen discovery technology. Combining this technology with CureVac’s general vaccine technology could create a panel of neo-antigens that could be used across many more patients, a spokesman for CureVac claims.
The timescales for getting a cancer mRNA vaccine to the clinic are hard to predict. But at Moderna, Aanur says the company hopes to have an update on its proof-of-concept personalised cancer programme later in 2022.
Meanwhile, CureVac’s Gnad-Vogt remains tight-lipped on timeframes but says that, as well as the vaccines currently under evaluation, the company hopes to enter phase I trials with a second-generation technology in 2022. This technology — which is already used to make an updated COVID-19 vaccine in collaboration with GSK[8] — optimises the nucleotide sequence of the mRNA to make proteins for a longer period, triggering a stronger immune response, among a few other tweaks.
mRNA vaccines are only going to get more exposure. For those who have been in the field for the past 30 years, the success of the COVID-19 vaccines is a pivotal part of the story. Lyerly says: “Twenty years ago, people said, ‘This is a stupid idea. Why would anybody do this?’ And now it’s, ‘This seems like such a good idea. Let’s do this’.”
Read more: Understanding mRNA vaccine technologies
- 1Boczkowski D, Nair S, Nam J-H, et al. Induction of Tumor Immunity and Cytotoxic T Lymphocyte Responses Using Dendritic Cells Transfected with Messenger RNA Amplified from Tumor Cells. Cancer Res 2000;60:1028–1034.https://aacrjournals.org/cancerres/article/60/4/1028/507052/Induction-of-Tumor-Immunity-and-Cytotoxic-T
- 2Bonehill A, Tuyaerts S, Van Nuffel AM, et al. Enhancing the T-cell Stimulatory Capacity of Human Dendritic Cells by Co-electroporation With CD40L, CD70 and Constitutively Active TLR4 Encoding mRNA. Molecular Therapy. 2008;16:1170–80. doi:10.1038/mt.2008.77
- 3Van Lint S, Renmans D, Broos K, et al. Intratumoral Delivery of TriMix mRNA Results in T-cell Activation by Cross-Presenting Dendritic Cells. Cancer Immunology Research. 2016;4:146–56. doi:10.1158/2326-6066.cir-15-0163
- 4Morse MA, Lyerly HK. Checkpoint blockade in combination with cancer vaccines. Vaccine. 2015;33:7377–85. doi:10.1016/j.vaccine.2015.10.057
- 5Lopez JS, Camidge R, lafolla M, et al. CT301 – A phase Ib study to evaluate RO7198457, an individualized Neoantigen Specific immunoTherapy (iNeST), in combination with atezolizumab in patients with locally advanced or metastatic solid tumors. Virtual: 2020.
- 6Delord J-P, Block MS, Ottensmeier C, et al. Phase 1 studies of personalized neoantigen vaccine TG4050 in ovarian carcinoma (OC) and head and neck squamous cell carcinoma (HNSCC). JCO. 2022;40:2637–2637. doi:10.1200/jco.2022.40.16_suppl.2637
- 7Balachandran VP, Rojas LA, Sethna Z, et al. Phase I trial of adjuvant autogene cevumeran, an individualized mRNA neoantigen vaccine, for pancreatic ductal adenocarcinoma. JCO. 2022;40:2516–2516. doi:10.1200/jco.2022.40.16_suppl.2516
- 8Gebre MS, Rauch S, Roth N, et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature. 2021;601:410–4. doi:10.1038/s41586-021-04231-6