Alamy Stock Photo
It is estimated that around one-third of people with COVID-19 do not have symptoms and could be spreading the virus unknowingly. To help tackle this, the government has rolled out rapid lateral flow antigen tests that can detect cases in under 30 minutes, meaning those who have a positive test can isolate immediately.
However, the accuracy of these tests — which are available from community pharmacies in England — and the value of this kind of mass testing is currently a topic of debate. We weigh up the current evidence around the reliability of COVID-19 lateral flow tests and what pharmacists need to know about them.
Sign in now if you’re an RPS member or subscriber.
Become a member of the RPS or subscribe to gain unlimited access.
What are lateral flow antigen tests?
Lateral flow antigen tests are tests that can process COVID-19 samples quickly without the need for laboratory equipment. They differ from polymerase chain reaction (PCR) tests, which look for genetic material from the virus and are generally more sensitive.
The benefit of lateral flow tests is that most tests generate easy-to-understand results in under half an hour and can be used at the point of care rather than sent to a lab to process.
In December 2020, the Medicines and Healthcare products Regulatory Agency (MHRA) issued an exceptional use authorisation (EUA) to the Department of Health and Social Care to allow the use of the Innova lateral flow self-test to detect infection in asymptomatic individuals who otherwise would not be tested.
An EUA is when a manufacturer applies to supply a medical device that does not comply with the law to protect a patient’s health if there is no legitimate alternative available.
The EUA means that the Innova lateral flow test can be used by a member of the public, with no previous experience of testing, in their own home or another community setting such as a place of work.
How does the test work?
Lateral flow is an established technology, adapted to detect the presence of a particular protein target, and is routinely used in healthcare settings because it is affordable, easy to use, delivers fast results and has a high level of accuracy. The best-known example of a lateral flow test is the home pregnancy test kit.
In the case of COVID-19, the target of the lateral flow test is proteins, or antigens, found in the COVID-19 virus.
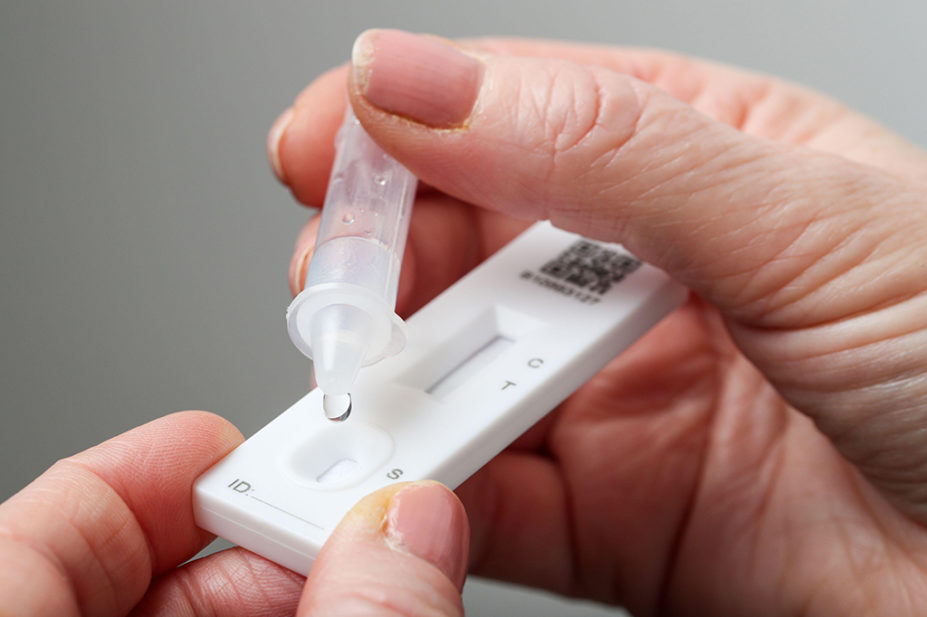
The COVID-19 test kit is a hand-held device with an absorbent pad at one end and a reading window at the other. Inside the device is a strip of test paper that changes colour in the presence of COVID-19 antigens.
Using one swab, a sample is taken first from the tonsils and then from the nostril. Alternatively, a saliva sample may be used.
What do the results mean?
If you get a positive result from a lateral flow test, it is extremely likely you are currently infected with COVID-19 and risk infecting others.
If you get a positive result, you will need to report your result on the government website and take a PCR test to confirm the result. You and anyone you live with will also need to self-isolate immediately.
If you get a negative result from these self-tests, it means that the test has not been able to detect the COVID-19 virus in your sample. However, you may still be infectious, so you must still follow rules regarding social distancing and face coverings.
Are the tests 100% reliable?
No. The MHRA has stated that “no test is 100% reliable”, even those that meet regulatory standards for performance and safety.
Therefore, it has emphasised that lateral flow tests are only authorised to be used as a “red light” test in order to find infectious people and ensure they self-isolate quickly, and not as a “green light” for people who test negative to enjoy greater freedoms.
This is because, unlike PCR tests, lateral flow tests cannot detect very low levels of coronavirus in a sample. This means the test may not give a positive result if you have only recently been infected; are in the incubation period; or if you have mostly recovered.
The accuracy of lateral flow tests is also dependent on the person who does the test. Training of supervisors at lateral flow test sites and regular use of the tests will help to mitigate this, as people become more proficient in using lateral flow tests.
What evidence is there regarding the effectiveness of lateral flow antigen testing?
A Cochrane review, published on 24 March 2021, investigated whether commercially available, rapid point‐of‐care antigen (lateral flow) and molecular tests were accurate enough to diagnose COVID‐19 infection reliably, and to find out if accuracy differed in people with and without symptoms.
The reviewers included 64 studies published up to September 2020, which investigated 16 different antigen tests and five different molecular tests.
They found that in people with confirmed COVID‐19, antigen tests correctly identified COVID‐19 infection in an average of 72% (ranging from 34% to 88%) of people with symptoms, compared with 58% of people without symptoms. In addition, they found that the tests were most accurate when used in the first week after symptoms developed.
In people who did not have COVID‐19, antigen tests correctly ruled out infection in 99.5% of people with COVID-19-like symptoms and 98.9% of people without symptoms.
Although the overall results for diagnosing and ruling out COVID-19 were good, the reviewers pointed out that 69% of studies used the test in laboratories instead of at the point of care.
They concluded that some lateral flow antigen tests may be most useful to identify outbreaks, or to select people with symptoms for further testing with PCR, allowing self‐isolation or contact tracing, and reducing the burden on laboratory services. But they added that more evidence was needed on rapid testing in people without symptoms.
Analysis of community testing data, released by the Department of Health and Social Care (DHSC) in March 2021, suggested that lateral flow tests had a specificity of “at least 99.9%”. Specificity is a measure of how good the test is at detecting true negative cases; in this case, a 99.9% specificity means that there is fewer than one false positive in every 1,000 lateral flow tests carried out.
However, the analysis used data from locations using the supervised testing model.
Clinical evaluation carried out by Public Health England (PHE) and the University of Oxford at the end of 2020 showed that lateral flow tests were accurate enough to be used in the community, including for asymptomatic people, but that the tests performed best when levels of virus are at their highest and people are in the most infectious stage of the disease.
How often should we be tested?
According to the DHSC, testing using lateral flow tests should be a “regular habit”.
Regular, rapid testing has been in place for some time for NHS and care home staff. The government now recommends twice-weekly testing using lateral flow tests for free to all adults in households with primary- and secondary-school, and college-aged children and young people, including childcare and support bubbles. This is in addition to the two tests that all secondary-school and college students and staff are asked to carry out each week at home.
Is there any difference between the different manufacturers’ versions?
The Cochrane review mentioned above found that different brands of tests varied in accuracy, and that there was a need for more studies directly comparing one test brand with another.
The Innova lateral flow test, which is provided via the Pharmacy Collect service in England and at COVID-19 testing sites, was found to have an average sensitivity (the proportion of people with a disease that have a positive test) of 57.5% and an average specificity (a measure of how good the test is at detecting true negative cases) of 99.6%.
The performance of the Innova test in asymptomatic people was not included in the analysis as studies have yet to be published.
PHE Porton Down’s labs have shown four lateral flow tests to have a sensitivity of more than 70% for all PCR positive cases but, importantly, they are able to catch those with high viral loads, suggesting they are effective in identifying cases that are most likely to transmit the disease.
Other lateral flow tests are also available from third-party providers who have declared that they meet the government’s minimum standards, but the government does not endorse or recommend any particular private testing providers.
In May 2021, the DHSC launched a consultation to collate views about the validation regime for COVID-19 tests, including lateral flow tests. Responding to the consultation the Royal Pharmaceutical Society (RPS) called for there to be a validation process for private COVID-19 tests before they are made available to purchase, so they undergo quality and accuracy checks to ensure they meet all safety requirements.
Where can I get a lateral flow test?
Up to two packs of seven lateral flow testing kits can be ordered, for free, on the UK government website. Alternatively, people can collect kits from a test site or, in England, from community pharmacies under the Pharmacy Collect scheme.