Centre for Infections / Public Health England / Science Photo Library
On 13 June 2012, a patient presented at Dr Soliman Fakeeh Hospital in Jeddah, Saudi Arabia, with what seemed like pneumonia and renal failure. The patient died 11 days later.
Virologist Ali Mohamed Zaki, who worked in the hospital’s diagnostic laboratory, suspected that sputum samples obtained from the patient contained a virus. But it was not until several months later, with a spate of patients across the Middle East reporting similar respiratory symptoms, that he confirmed his hunch. The illness was caused by a novel coronavirus, dubbed Middle East respiratory syndrome coronavirus (MERS-CoV).
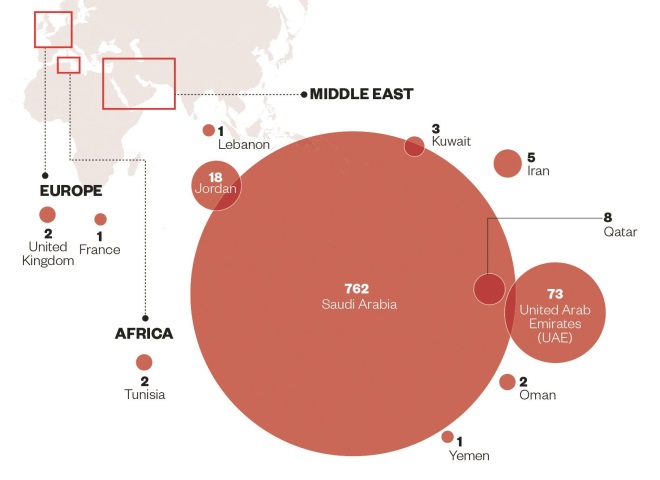
Source: European Centre for Disease Prevention and Control
Number of confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) that were locally infected
Coronaviruses are a species that often infect humans and are one of the causes of the common cold. “The reason there is no specific treatment is that coronaviruses used to be so mild that nobody really cared,” says Jasper Chan, a microbiologist at the University of Hong Kong.
The emergence of the highly contagious severe acute respiratory syndrome (SARS) coronavirus in Vietnam in February 2003 proved these airborne viruses can turn deadly. Within a few months, the virus spread to infect over 8,000 people in 37 countries, with a 9.6% death rate.
But in 2004, when the SARS virus disappeared just as suddenly as it had arrived, most research that had been kick-started was quietly halted. “SARS renewed interest in research,” says Chan, “but [the outbreak] lasted for less than a year, so all subsequent findings in terms of potential targets ended up pre-clinical, because there were no more human cases.”
Unlike SARS, however, MERS-CoV is yet to go away. As of 13 October 2014, 896 cases of MERS-CoV have been reported to public health authorities worldwide, including 357 deaths — 40% of patients die within 30–40 days of the onset of symptoms. Most of the cases have been in the Middle East, with hundreds of new cases in Saudi Arabia and the United Arab Emirates in spring 2014. Cases outside of the Middle East — most recently in Austria on 29 September 2014 — occur in people who have travelled there or who have come into contact with an infected person who has travelled there.
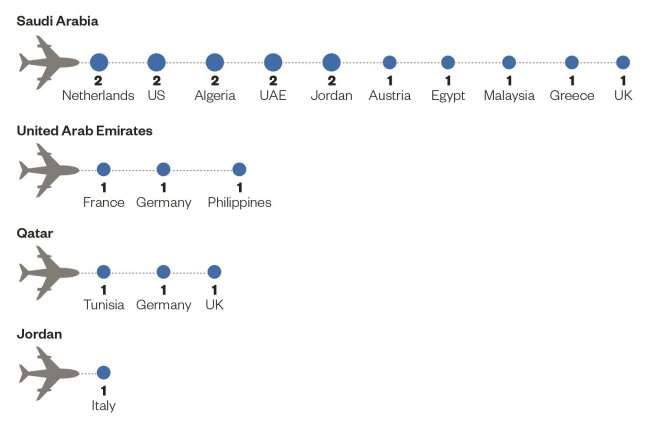
Source: European Centre for Disease Prevention and Control
Imported cases and place of probable infection with the Middle East respiratory syndrome coronavirus (MERS-CoV)
With millions of Muslims visiting Saudi Arabia for the Hajj pilgrimage each October, the fear is that the virus — which has no effective vaccine or treatment — could rapidly spread beyond the region, costing countless human lives. Researchers are investigating a variety of methods to combat the virus, from using combinations of approved drugs to experimental vaccine strategies.
Treatment options
In the spring of 2013, there were three individuals suffering from MERS-CoV in three different intensive care units in hospitals in England. Government agency Public Health England (PHE) and the International Severe Acute Respiratory and Emerging Infection Consortium published a decision support tool in June 2014, which lists existing therapies that are plausible for treating the infection and are supported by reasonable in vitro, animal or clinical data from MERS-CoV or other respiratory virus infections[1]
.
The very first case of MERS diagnosed during an individual’s life occurred in London in September of 2012. The patient was on an intensive care unit in London for nine months, and eventually died.
“Part of our thinking was that we needed to provide a rational approach for [intensive care doctors] who are not coronavirus experts,” says Maria Zambon, director of reference microbiology at PHE.
“The very first case of MERS diagnosed during an individual’s life occurred in London in September of 2012. The patient was in an intensive care unit in London for nine months, and eventually died,” recalls Zambon.
The guidance divides potential therapies for MERS-CoV into green, yellow, and red groups. Green indicates that the benefit is likely to exceed the risks, yellow indicates that the data are inadequate for assessment and red indicates the risk is likely to exceed the benefit. Among the green treatments are convalescent plasma, interferons, lopinavir, and polyclonal and monoclonal antibodies.
PHE notes that “interferon and lopinavir are likely to be the most accessible initial treatments”. However, it advises that treatment with specific drugs should ideally occur in the context of formal observational studies or controlled intervention trials. Zambon stresses that first-line care for MERS-CoV patients is high quality supportive care (for example, fluid management and mechanical ventilation where necessary). “Then, you have to decide, are you going to try something?” There is a lot of discussion among infectious disease doctors in Britain as to when it is appropriate to try active treatment for MERS-CoV patients, says Zambon.
Assessing the risks
“MERS is very severe clinically, not only affecting the lungs, and patients with co-morbidities or who are of an older age should be considered for treatment,” says Chan.
However, David Heymann, an epidemiologist at the London School of Hygiene and Tropical Medicine, argues that there is no need at present to provide treatments that are not approved for use in the condition. “There has not been a recorded MERS case among Hajj pilgrims in the past two years. The outbreak was based in hospitals, where [the virus] was transferred by poor hospital practices,” he says.
Heymann explains that improved hygiene practices in hospitals in Saudi Arabia, along with a large training programme to educate health workers about how to deal with the virus that began in April 2014, has led to the outbreak being largely contained. But there’s always a chance that the virus will mutate, he concedes.
There appears to be little consensus on the risk posed by MERS-CoV. “The Kingdom of Saudi Arabia and the United Arab Emirates had a lot of trouble controlling the outbreak this year,” said Allison McGeer, director of infection control at Mount Sinai Hospital in Toronto, during her presentation on MERS-CoV at the Interscience Conference on Antimicrobial Agents and Chemotherapy, held in Washington, DC, in September 2014.
“There were many more cases this year than last. There were also more cases in April and May of the last two years and, since that matches camel birthing season, one hypothesis is that they might be related,” said McGeer, who has treated MERS-CoV patients in Saudi Arabia.
McGeer said the virus is widespread in camels and explained that in some regions, 93% of adult camels and 55% of juveniles are seropositive for MERS-CoV.
Novel ideas
Molecular biologist Eric Snijder and his colleagues at Leiden University in the Netherlands are one of several research teams experimenting with existing medicines to see if they can identify effective treatments.
“When you get infected with a new organism, it takes seven to ten days to mount an immune response. So there’s a window of opportunity for getting the immune system started. If an agent is partially active, it can help get a patient through the worst week of the infection,” says Snijder.
Snijder’s team has identified four agents — chloroquine, chlorpromazine, loperamide, and lopinavir — out of 348 Food and Drug Administration-approved compounds[2]
.
Since MERS-CoV is lethal and could theoretically disappear suddenly like SARS, it is important to find treatments that work now, rather than waiting ten years for new drugs to come through clinical trials. The next step for the agents selected by Snijder’s team is to test them in an animal model.
Snijder says his team has conducted a further screening that identified another dozen existing agents which have similar activity. He expects to publish the results of that screening in 2015.
Human data on experimental drugs or off-label use of licensed drugs is either sparse or emerging. “Researchers have already published an article on the use of interferon and ribavirin in five MERS patients in the Middle East[3]
. Five died, but does it mean that ribavirin is not effective? We don’t know because they were all older people, who received the drugs after at least two weeks of symptom onset,” says Chan in Hong Kong.
“We also have some clinical data with the HIV drug lopinavir from the SARS epidemic,” he adds. A study compared 41 SARS patients treated with lopinivir with 111 historical controls. Of the controls, 6.3% (or 7 patients) died within 21 days, compared with none of the treatment group. Rates of acute respiratory distress syndrome were also lower in those treated with lopinivir — 2.4% compared with 22.5% among the historical controls[4]
.
Chan is currently collaborating with the University of Michigan on an experimental antiviral called BanLec, which has shown in vitro efficacy against MERS-CoV. Chan’s group has also developed an antiviral peptide, HR2P, with a six-helix bundle fusion core structure of the corona virus’s spike protein, which has shown a potent inhibitory effect against MERS-CoV[5]
.
Hiding place
MERS-CoV is an enveloped, single-stranded, positive-sense RNA virus. “RNA viruses are out-competing us, as scientists and politicians struggle to keep up with them,” says Snijder .
RNA viruses are out-competing us, as scientists and politicians struggle to keep up with them.
“They are a special group of organisms with rapid evolution. The flu, a good example of an RNA virus, is a quasispecies, meaning that the flu vaccine needs to be repeated every year because last year’s vaccine is not adequate,” he explains.
It is thought that many species of virus reside in animal reservoirs, jumping the species barrier into humans from time to time. There is some evidence that the single-humped dromedary camel harbours MERS-CoV, although the majority of cases are linked to hospital outbreaks. Genomic sequencing of the virus has shown that the closest relatives are bat coronaviruses.
An alternative to using off-label medicines in humans is to tackle one of the potential sources of human infection. At the University of Pittsburgh, Pennsylvania, vaccine researcher Andrea Gambotto is trying to develop a way to protect camels against infection, and potentially deplete a reservoir of the virus.
“The question is whether the camel is the only animal that is infected by MERS,” says Gambotto. “The animal reservoir usually works in combination, with two animals in close relation who ping-pong the virus, like tick and deer for lyme disease. You need both animals for the virus to survive.”
Developing a camel vaccine is an attractive strategy, not only because it avoids the immense financial cost of developing a human vaccine, but because regulatory hurdles that apply to investigatory veterinary medicine are vastly decreased in the countries with index cases.
Gambotto and colleagues have created two genetically recombinant candidate adenovirus vectors encoding the full-length MERS-CoV S protein and the S1 extracellular domain of S protein. Mice were immunised with both candidate vaccines intramuscularly, with an intranasal booster three weeks later. All of the mice showed an antibody response, which neutralised MERS-CoV in vitro
[6]
.
“Next we would like to do an experiment in a field where MERS is circulating, and try to vaccinate camels,” says Gambotto.
Whether it is initiating public health measures, investigating existing drugs or developing new ones, if MERS-CoV disappears as suddenly as SARS, it is possible that substantial investment in finding a way to combat the disease will subside too. And how long will it be before the next fatal coronavirus emerges?
References
[1] Public Health England/International Severe Acute Respiratory and Emerging Infection Consortium. MERS-CoV: clinical decision making support for treatment. July 2014. Available at: https://www.gov.uk/government/publications/mers-cov-clinical-decision-making-support-for-treatment (accessed 18 October 2014).
[2] De Wilde AH, Jochmans D, Posthuma CC et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrobial Agents Chemotherapy 2014;58:4875–4884.
[3] Al-Tawfiq JA, Al Rabiah F, Khan B et al. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. International Journal of Infectious Disease 2014;20:42–46.
[4] Chu CM, Cheng VCC, Hung IFN et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004;59:252–256.
[5] Lu L, Liu Q, Zhu Y et al. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nature Communications 2014, online 28 January 2014. doi: 10.1038/ncomms4067.
[6] Kim E, Okada K, Kenniston T et al. Immunogenicity of an adenoviral-based Middle East Respiratory Syndrome coronavirus vaccine in BALB/c mice. Vaccine 2014;32:5975–5982.
You may also be interested in
Norovirus and strategies for infection control

Case-based learning: insect bites and stings
