David Scharf / Science Photo Library
On 17 October 2007, billionaire and philanthropist Bill Gates and his wife Melinda stood before a group of 300 malaria researchers, funders and policymakers at the Sheraton Seattle Hotel, Washington, and asked them to reconsider a proposition that had been shelved for nearly four decades: malaria eradication.
“To aspire to anything less is just far too timid a goal for the age we’re in. It’s a waste of the world’s talent and intelligence, and it’s wrong and unfair to the people who are suffering from this disease,” Melinda Gates said.
This bold proposal to stop transmission and end the disease would require new tools, she added — new medicines, new insecticides or a new vaccine. Researchers had been working to develop malaria vaccines for decades but the primary goal had been to prevent illness and deaths, not necessarily to eliminate the parasite. This new commitment to malaria eradication would shift the vaccine developers’ focus from combatting the disease, which led to 584,000 deaths in 2013[1]
, to halting the spread of the parasite.
The announcement was a shot in the arm for the handful of researchers who had been trying to develop transmission-blocking vaccines. These vaccines are not designed to block infection in the person who receives the shot. Instead, they stop the immunised individual from transmitting the parasite. So when a mosquito bites a vaccinated individual, the insect does not become infected and the next person it bites does not contract the disease. The strategy raises a number of questions surrounding the evidence that would be needed to gain regulatory approval and the ethics of administering a vaccine that will not provide immediate benefit.

Ashley Birkett, director of the PATH Malaria Vaccine Initiative, says the transmission-blocking vaccines would have a delayed benefit to humans
Some people call transmission-blocking vaccines altruistic vaccines. But that’s not quite right, says Ashley Birkett, director of the PATH Malaria Vaccine Initiative (MVI), based in Washington, DC. “We view it as a delayed benefit,” he says. “It would help the individual, but it wouldn’t necessarily help them immediately.”
Before the meeting in Seattle, the approach hadn’t gained much attention. “It wasn’t clear that the field was particularly supportive,” recalls Carole Long, a malaria researcher at the US National Institute of Allergy and Infectious Diseases (NIAID) in Rockville, Maryland. But now that eradication is back on the table, the unorthodox idea is gaining traction. Gates’s call to arms “totally changed the whole field,” says Robert Sauerwein, a malaria vaccine researcher at the Radboud University Medical Center in Nijmegen, the Netherlands. “Now [the idea] is mainstream.”
According to a 2013 report by Policy Cures, an independent non-profit research group, there has been a five-fold increase in annual funding for malaria research and development over the past two decades — from US$131m in 1993 to US$610m in 2011. And funding may increase even further. At the annual meeting of the American Society of Tropical Medicine and Hygiene in November 2014, Bill Gates announced that the foundation has boosted its malaria programme budget by 30% to more than US$200m per year. The foundation also awarded a US$156m grant to the PATH Malaria Vaccine Initiative to advance the development of next-generation malaria vaccines to help eliminate the disease. At least some of that funding will go toward further development of transmission-blocking vaccines.
The pool of researchers working to develop a transmission-blocking vaccine has expanded too. And they are making slow but steady progress towards a vaccine they hope will help wipe out the parasite for good.
Parasite bottlenecks
A vaccine that doesn’t protect the person who receives it may seem counterintuitive, but there are good reasons for pursuing such a vaccine. To understand the rationale, at least a cursory understanding of the parasite’s complicated life cycle is needed (see figure). When an infected mosquito bites a human, the parasite travels from the insect’s salivary glands into the bloodstream. From there, the parasite makes its way into the liver cells, where it multiplies for about a week. Then it once again enters the bloodstream and begins to invade red blood cells. Within these cells, the parasite multiplies again and again, until the blood teems with billions of parasites. Some of the parasites that enter red blood cells transform themselves into male and female parasites called gametocytes. It’s the gametocytes that travel from a human to a mosquito. When a mosquito feeds on an infected person, the gametocytes hitch a ride into the mosquito’s gut. They fuse, and the cycle begins again.
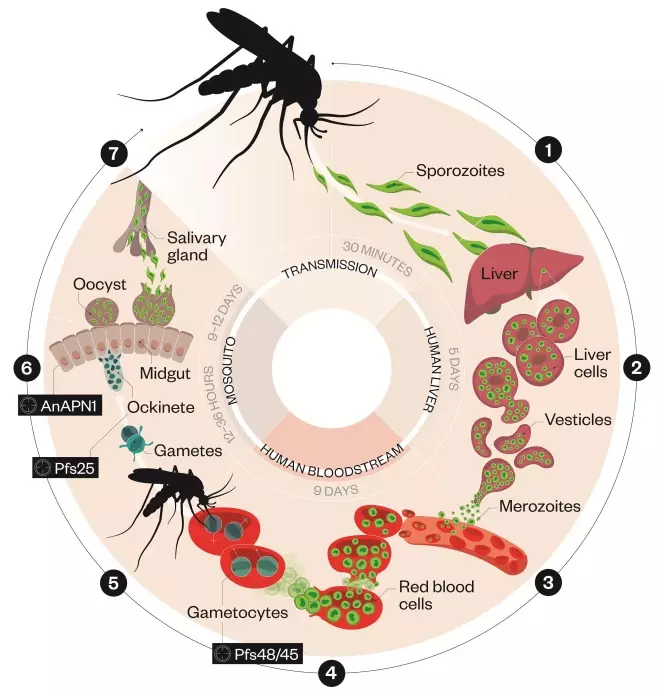
Malaria parasite lifecycle
Source: PATH Malaria Vaccine Initiative; Medicines for Malaria Venture / Inforgraphic: Alisdair Macdonald
1) An infected Anopheles mosquito bites a person, transmitting the Plasmodium falciparum parasite, in the form of sporozoites, into the bloodstream. Sporozoites reach the liver in just 30 minutes.
2) After reaching the liver, the sporozoites multiply within liver cells causing no symptoms.
3) The sporozoites become merozoites, which re-enter the bloodstream and invade red blood cells. Inside these cells they multiply asexually until the cells burst, allowing the newly formed merozoites to invade more red blood cells. This cycle, which takes about 43–48 hours, causes the hallmark fever and chills.
4) Some merozoites develop into male and female parasites, called gametocytes, that remain in red blood cells.
5) When a mosquito bites an infected person, the gametocytes are transferred to the mosquito’s gut, where they develop into mature sex cells called gametes.
6) The fertilized female gamete forms ockinetes that burrow through the mosquito’s gut wall and form oocysts on the exterior surface.
7) Inside the oocysts, thousands of sporozoites develop. These sporozoites are released into the body cavity of the mosquito and travel to its salivary glands.
If the goal is to stop the spread of malaria, a vaccine must either prevent mosquitoes from infecting humans or prevent humans from infecting mosquitoes. “We see those bottlenecks as really good places to target,” says Birkett. That’s because the number of parasites is small, with perhaps a few dozen parasites travelling from the mosquito to the human. “And when you go from a human to a mosquito, it’s probably as low as one to three parasites,” he says.
PATH’s MVI has been funding research into both types of vaccines, and it’s had some success. The leading vaccine candidate, RTS,S, which could be given the green light by the European Medicines Agency in 2015, aims to induce immune responses that prevent malaria parasites from invading liver cells. However, it’s only partially effective. While the vaccine has been shown to nearly halve the number of symptomatic malaria cases in young children, it only cuts infection in children by 30%. And even a child who doesn’t develop symptoms of malaria may still have malaria parasites, which can contribute to the spread of the disease. “[RTS,S] is probably going to save a lot of lives,” says Rick King, director of research and development at MVI, “but it’s not going to be sufficiently potent to be part of a campaign to eliminate malaria.”
Source: BSIP SA / Alamy
An experimental vaccine aims to protect mosquitoes from parasite in human bloodstream
Even if better vaccines come along in the future, it is unlikely they will be able to totally block transmission from mosquitoes to humans. As the parasite passes through each stage of its life cycle, it morphs into a new version of itself and the proteins that coat its surface change. The proteins that cover the parasite when it’s in the blood, and even the liver, can be variable because the parasite must constantly evolve to evade the human immune system. A vaccine that targets one of these proteins might not work against a slightly different version of that same protein.
This is why some researchers are working to develop vaccines that block transmission from humans to mosquitoes. The proteins that coat the parasite in the mosquito never interact with the human immune system, so they don’t face the same selection pressure. “There’s little genetic diversity,” says Teun Bousema, a malaria epidemiologist at the Radboud Institute for Health Sciences, so “it should be a little bit more straightforward to come up with an efficacious vaccine”. Rhoel Dinglasan, a malaria researcher at the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland, points out another advantage: parasites invade the liver rather quickly, in about 30 minutes. But it takes nearly a day for the parasite to infect a mosquito, so the window of opportunity is much longer.
Pfs25 has been at the forefront of the handful of candidates that are of interest for a transmission-blocking vaccine
Target practice
While most researchers recognise that a transmission-blocking vaccine would be a valuable tool in the fight to eradicate malaria, there is little consensus on what the vaccine should look like. The most advanced candidate, developed by NIAID, combines a surface protein on the Plasmodium falciparum parasite called Pfs25 with bacterial toxin that has been tweaked to be non-toxic. “Pfs25 has been at the forefront of the handful of candidates that are of interest for a transmission-blocking vaccine,” says Patrick Duffy, chief of the Laboratory of Malaria Immunology and Vaccinology at NIAID. The drawback is that the protein tends to elicit a very modest immune response. To help boost the response, Duffy and his colleagues attached the surface protein to a bacterial protein. The team also added an adjuvant to boost the immune response even further. The hope is that the vaccine will prompt the immunised individual to churn out antibodies against Pfs25. Those antibodies will not block infection because the form of the parasite that mosquitoes transmit to humans does not express this protein on its surface. However, if that person contracts malaria and then gets bitten by a mosquito, the mosquito will ingest not only malaria parasites, but also the Pfs25 antibodies. After the gametocytes fuse, Pfs25 becomes the major surface protein. It’s not entirely clear what role Pfs25 plays in the parasite’s life cycle, but antibodies against the protein seem to prevent the parasite from migrating out of the mosquito’s gut, a key step in its development.
Vaccine Targets
Pfe25: A vaccine candidate targets Pfs25, a protein on the parasite’s surface. When the mosquito ingests Pfs25 antibodies from a vaccinated person, it prevents the parasite from migrating out of the mosquito’s gut.
AnAPN1: A vaccine candidate targets the enzyme AnAPN1 in the gut of the mosquito, which plays a key role in the ability of the malaria parasite to infect mosquitoes.
Pfe48/45: A vaccine candidate targets Pfs48/45, a protein expressed on the surface of gametocytes, and glutamate-rich protein (GLURP), found on the form of the parasite that invades red blood cells. The Pfs48/45 portion stops fertilisation in the mosquito gut and the GLURP portion reduces the number of parasites circulating in the blood.
The team tested the vaccine in a safety study at Johns Hopkins University in 2011. They enrolled 30 healthy volunteers and administered up to three doses of the vaccine[2]
. To test the vaccine’s activity, the researchers collected sera from the vaccinated volunteers, mixed it with gametocytes in the laboratory and allowed mosquitoes to feed on the mixture. The goal was to reduce the number of parasites in the mosquito by at least half. “We observed that antibody levels were increasing after each dose,” says Duffy. But even after the third dose, “only a few individuals had activity that reduced parasite burden by greater than half”. So the researchers convinced 11 of the participants to receive a fourth injection. “The results were very encouraging,” says Duffy. None of the participants experienced any serious side effects. And 9 of the 11 volunteers who received a fourth dose achieved high enough antibody levels to reduce the parasite burden in the mosquitoes by more than 50%.
In 2013, Duffy and his team partnered with scientists in Mali for a second trial in a village called Bancoumana, where malaria is transmitted during the rainy season. The researchers have already administered four shots of the vaccine to about 50 volunteers, but they won’t have the results until April 2015. “What we can say is that the vaccine was very well tolerated,” says Duffy. The researchers plan to test the participants’ blood in the laboratory as they did in the first study, but they’re also attempting a more realistic way to measure the vaccine’s activity. After the fourth dose, the researchers allowed uninfected mosquitoes to feed on the participants once a week for six weeks. In both cases, they’ll be looking to see whether the mosquitoes are infected and, if so, how many parasites they have. Participants in the trial were randomised to receive either the malaria vaccine or vaccines against hepatitis B and meningococcal disease. The hope is that the group that received the malaria vaccine will infect fewer mosquitoes than the group that did not.
“We see this trial as the first step in a series of trials where we’re putting in place the tools that will allow us to measure vaccine activity, seeing if we can measure vaccine activity and then determining how we can improve on that vaccine activity,” says Duffy. The next step is to combine Pfs25 with a second antigen, Pfs230. “We’re going to try to attack both phases of development in the mosquito with that combination vaccine,” he says.
We’re going to learn much more about the kinds of immune response we need to elicit in order to get transmission blocking
At least one other transmission-blocking vaccine is currently being tested in humans. In 2013, the Fraunhofer Center for Molecular Biotechnology in Plymouth, Michigan, launched a small safety study of another vaccine that uses Pfs25. Instead of hooking the protein to a bacterial toxin, the researchers attached it to the surface of a structure that resembles a virus. “The idea there is that the immune system might be particularly well adapted to recognising virus particles and mounting antibody responses to those,” says King. These studies are meant to show proof-of-concept. “We’re going to learn much more about the kinds of immune response we need to elicit in order to get transmission blocking,” he says.

Source: Chris Hartlove
Rhoel Dinglasan, a malaria researcher at the John Hopkins Bloomberg School of Public Health, and his colleagues have shown that AnAPN1 antibodies can block mosquitoes from contracting both Plasmodium falciparum and Plasmodium vivax
Pfs25 vaccines have been tested in the clinic before and failed, either because they couldn’t provoke an immune response or because they caused adverse reactions. So some researchers have set their sights on other targets. Dinglasan has developed a vaccine that targets an enzyme in the gut of the mosquito rather than a protein on the parasite. The enzyme, AnAPN1, helps mosquitoes digest blood, but it also appears to play a key role in the ability of malaria parasites to infect mosquitoes. While Pfs25 vaccines are designed to protect against P. falciparum, the most common malaria parasite in Africa, Dinglasan and his colleagues showed that AnAPN1 antibodies can block mosquitoes from contracting both P. falciparum and P. vivax, the parasite most common in Asia. When Dinglasan and his colleagues tested the vaccine in mice and non-human primates, it elicited a strong antibody response[3]
.
The blood stage gives you the personal protection and the sexual stage part gives you the population protection
Sauerwein has developed a vaccine candidate that may be able to block transmission and ward off the disease. To block transmission, the shot relies on a fragment of a protein called Pfs48/45, which is expressed on the surface of the gametocyte. When this protein is expressed in a laboratory, it has a tendency to fold incorrectly. But when the researchers linked it to a fragment of a protein called Glutamate-rich Protein or GLURP, they discovered they could coax Pfs48/45 into the right shape[4]
. GLURP is a protein found on the form of the parasite that invades red blood cells. A vaccine that elicits antibodies against both GLURP and Pfs48/45 could potentially reduce the number of parasites in the blood — which could prevent some of the worst symptoms of malaria — as well as reduce transmission to mosquitoes. “The blood stage gives you the personal protection and the sexual stage part gives you the population protection,” says Sauerwein.
Stumbling blocks
Even if the scientific challenges can be overcome, many logistical hurdles remain. For example, how would a company gain regulatory approval for this type of vaccine? The vaccine isn’t meant to prevent infection in an individual, but in a community. So a randomised trial that compares the number of infections in people receiving the vaccine with the number of infections in unvaccinated people wouldn’t work.
The default strategy, says Birkett, would be to compare the number of infections in different communities, some that receive the vaccine and others that don’t. These types of studies, called cluster randomised trials, have been done before, but they present many challenges, he says. Birkett and his colleagues recently examined the feasibility of conducting this kind of trial for a transmission-blocking vaccine. “It would require a very large number of clusters, probably over 50 clusters,” he says. The researchers would have to make sure that mosquitoes and humans don’t travel between the clusters, an undertaking that might prove exceedingly difficult. “So we think that the probability of having an error is quite high,” says Birkett. “Because of that, we’ve been exploring potential other regulatory pathways that might be more tractable.”
Another alternative might be to vaccinate an entire island. “Maybe you’d be able to show you could eliminate malaria from an island by implementing the transmission-blocking vaccine,” says Birkett. Or, if the vaccine qualified for accelerated approval, the bar for approval might be a bit lower. Perhaps the researchers would only need to prove that the vaccine prevents transmission from humans to mosquitoes to gain approval.
We really don’t know what it would take to gradually decrease the number of circulating parasites
It’s also unclear just how effective a transmission-blocking vaccine would have to be to reduce transmission. Sauerwein says he would hesitate to bring forward a vaccine that reduced the proportion of infected mosquitoes by less than 80%. But it’s also possible a 50% reduction would be sufficient to knock down transmission. “We really don’t know what it would take to gradually decrease the number of circulating parasites,” Long says.
Whether communities in Africa and elsewhere would be willing to accept a vaccine that provides only delayed protection also remains to be seen. Some vaccines developed in the West, such as the oral polio vaccine, have been met with suspicion, even when they provide protection against disease. But Bousema thinks a transmission-blocking vaccine would be welcome in many of the communities hardest hit by the disease. “There is a very clear indirect benefit,” he says. “If you manage to reduce transmission then you will protect yourself, your family and your neighbours.” But no vaccine is without risks, and it may be difficult to decide how much personal risk is acceptable when it’s the community that reaps the immediate benefit.
If you manage to reduce transmission then you will protect yourself, your family and your neighbours
Still, these vaccines could be a boon to the eradication effort. Tools such as insecticide-treated bed nets, indoor spraying and drugs can dramatically reduce malaria transmission. But when those interventions disappear, the parasite can come roaring back. “At some point you have to get rid of those last infections,” says Bousema. A transmission-blocking vaccine cannot eradicate malaria on its own, but it may be the weapon the world needs to deal malaria a death blow.
References
[1] World Health Organization. World Malaria Report 2014. December 2014.
[2] Talaat KR, Ellis RD, Durbin AP et al. Phase I trial of Pfs25-EPA/Alhydrogel a transmission blocking vaccine against falciparum malaria in healthy malaria-naïve adults. Abstract number 1455. ASTMH 61st annual meeting, November 2012.
[3] Jones RM, Chichester JA, Mett V et al. A plant-produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLOS ONE 2013;8: 79538.
[4] Michael Theisen M, Roeffen W, Singh SK et al. A multi-stage malaria vaccine candidate targeting both transmission and asexual parasite life-cycle stages. Vaccine 2014;32: 2623–2630.