At the start of the COVID-19 pandemic, hopes were pinned on vaccines achieving herd immunity and eventually wiping out the SARS-CoV-2 virus. But, as the virus quickly evolved, it became clear that this was not going to happen — the most vulnerable would need antiviral drugs to fall back on. The first generation of these antivirals enjoyed some success but have come with complications. Now, three years on, with COVID-19 still a leading cause of death in many parts of the world, there is a new generation of antivirals around the corner.
Some of these second-generation antivirals are simply improved versions of the first tranche; others are pursuing new targets that have been discovered since the start of the pandemic. “Therapeutics represent one of the last lines of attack,” explains Jay Luly, chief executive of Enanta Pharmaceuticals, a biotechnology company based in Watertown, Massachusetts, that develops antivirals. “We need to make sure that we have a strong armament in place.” The hope is that these new drugs will provide better protection, with fewer side effects, and, in the long term, be able to withstand viral evolution and the risk of resistance that comes with it.
First-generation fears
The first antivirals out of the gate were monoclonal antibody (mAb) drugs, which mimic human antibodies. They stick to the spike protein of the SARS-CoV-2 virus and neutralise it. In August 2021, the Medicines and Healthcare products Regulatory Agency (MHRA) approved REGEN-COV, a combination of two mAbs — casirivimab and imdevimab — developed by Regeneron Pharmaceuticals and Roche, for emergency use in the UK, with others following. But these mAbs must fit to the virus’s spike protein and, as the virus has mutated, their neutralising power has diminished. By November 2022, it was clear that most of the antibodies developed were not effective against the evolved Omicron variant of COVID-19, with UK guidance no longer recommending sotrovimab for routine use, and draft guidance from the National Institute for Health and Care Excellence rejecting three mAbs[1,2]. By December 2022, the US Food and Drug Administration withdrew authorisation from Eli Lilly’s bebtelovimab, the last remaining mAb that was considered effective.
The fact that vaccines struggled to keep current, and antibodies have dropped one by one, now puts therapeutics under the magnifying glass
Jay Luly, chief executive of Enanta Pharmaceuticals
“The fact that vaccines struggled to keep current, and antibodies have dropped one by one, now puts therapeutics under the magnifying glass,” says Luly.
When it came to therapeutics, most companies looked to strategies developed to fight other viruses. For example, Gilead’s remdesivir, which was originally developed to treat hepatitis C and subsequently tested for the Ebola virus, is a nucleotide analogue and mimics adenosine. When picked up by the virus to be incorporated into its RNA, it causes mutations, particularly blocking the action of the RNA-dependent RNA polymerase (RdRp) and preventing viral replication. “It seems to be effective when given very early,” says Martin Michaelis, pharmaceutical scientist at the University of Kent. “The downside is it needs to be given intravenously,” he adds, limiting its use to hospitalised patients.
The first COVID-19 oral antiviral to be approved — molnupiravir (Merck), which was approved by the MHRA in November 2021 — is also a nucleoside analogue. While these drugs are generally considered safe, there is some concern about their mutagenicity; “if it induces mutations, what does it do to the normal DNA in the cell?” questions Michaelis. Early trial results for molnupiravir were promising, showing about a 50% reduction in hospitalisation and death. However, these results were not backed up when more data were released in December 2021, in which hospitalisations and death were reduced to a disappointing 30%[3,4]. A study in December 2022 concluded that, although molnupiravir improved recovery times, it did not reduce the risk of hospitalisation or death in vaccinated, high-risk people infected with the Omicron variant[5].
The National Institute for Health and Care Excellence now says in draft guidance that neither remdesivir or molnupiravir are cost-effective and should not be used, leaving only Pfizer’s oral antiviral protease inhibitor Paxlovid, a combination of the drugs nirmatrelvir and ritonavir.
So far, Paxlovid administered for five days has not shown any resistance in the clinic …[but] it may change
Jean-Pierre Sommadossi, chief executive of Atea Pharmaceuticals
Nirmatrelvir targets the virus’s 3C-like protease, which is crucial for viral replication. Ritonavir is added to improve nirmatrelvir’s pharmacokinetics. On its own, nirmatrelvir is rapidly metabolised and eliminated, but ritonavir, a protease drug used in the treatment of HIV, inhibits its degradation and increases its half-life. “Pfizer did demonstrate very strong data,” says Luly. Pre-Omicron, Pfizer was able to demonstrate an up to 89% reduction in hospitalisation and death[6]. But there are significant limitations owing to ritonavir’s ability to boost other medicines a patient is taking, such as anticoagulants or immunosuppressive drugs. “So, a physician who is prescribing Paxlovid must weigh certain trade-offs of either stopping someone’s other medicine, or figuring out how to change the dose of another medicine in order to accommodate the ritonavir,” explains Luly. Given the demographic who are likely to be hospitalised, this is a major problem. “Those individuals have no treatment,” says Jean-Pierre Sommadossi, chief executive of Atea Pharmaceuticals in Boston, Massachusetts — a veteran of hepatitis C and HIV/AIDS antiviral development.
Another concern is the virus developing resistance to antivirals as it evolves. “So far, Paxlovid administered for five days has not shown any resistance in the clinic … [but] it may change,” says Sommadossi. The hope is that, in comparison to the spike protein, which has successfully mutated to avoid antibodies from vaccination, targeting a protease crucial to viral replication will limit its ability to successfully mutate.
The problem comes when patients who are immunocompromised harbour the replicating virus for weeks or months — in these cases, it is possible that resistance could emerge. In addition, the phenomenon of ‘viral rebound’, where seemingly COVID-free patients relapse after antiviral treatment, is now well known. “Nobody really knows exactly why [it happens],” says Luly, but he thinks it may signal that current antivirals are not able to reach all infected tissue.
Second-generation hopes
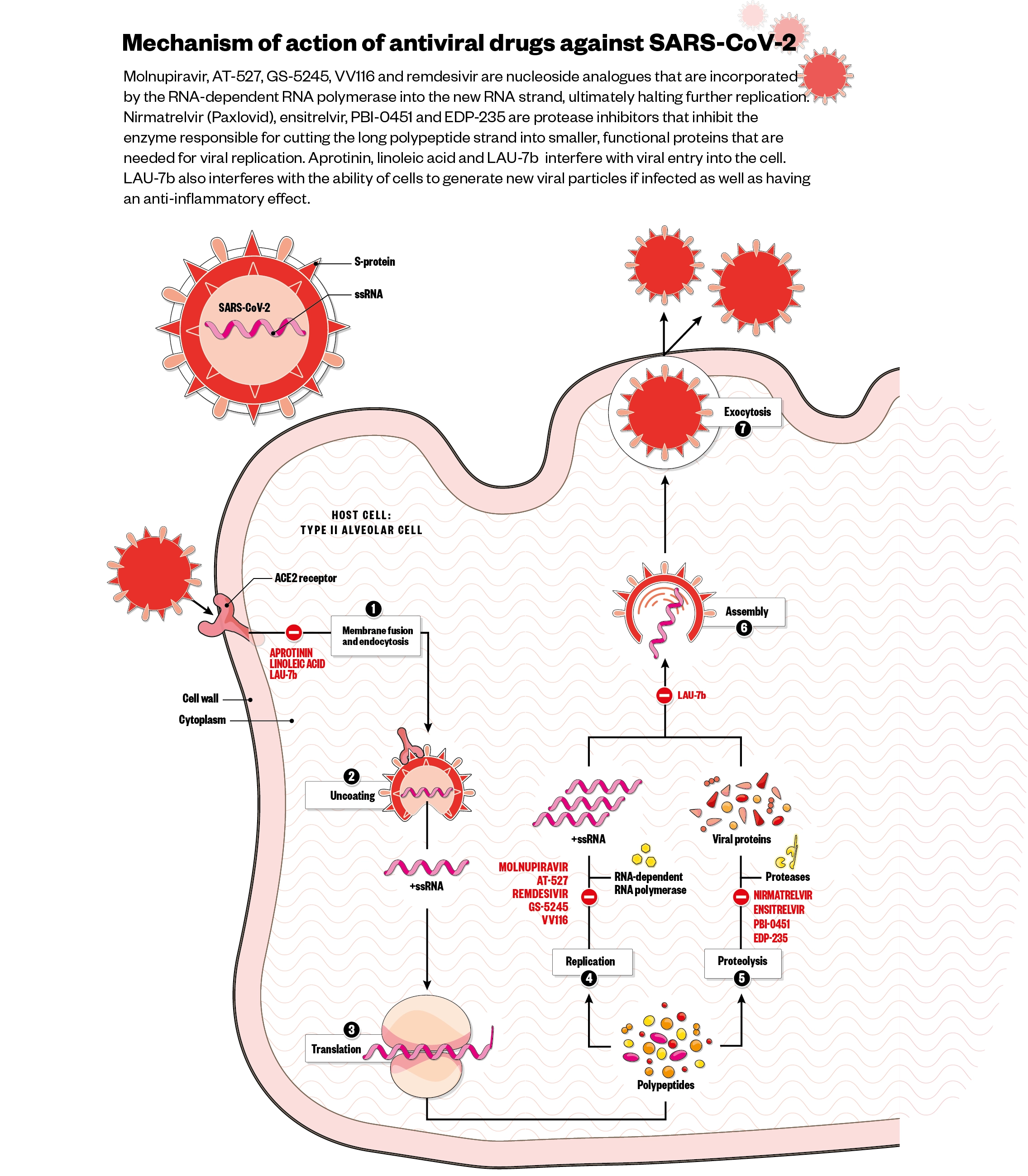
So, the hunt for new drugs continues (see Figure). Pfizer is actively pursuing second-generation antivirals; a spokesperson for the company says it is closely monitoring mutations and wants to “be at the ready, should resistance to our current COVID-19 antiviral develop”. Gilead has confirmed remdesivir has maintained its efficacy, but it has developed a novel oral nucleoside prodrug, called GS-5245, which is currently in a phase III trial for non-hospitalised patients who are at high risk of developing severe disease[7].
Chinese pharmaceutical companies Junshi Biosciences and Vigonvita Life Sciences are also co-developing an oral prodrug called VV116. A phase III study involving 771 participants, published in January 2023, concluded that, in adults with mild-to-moderate COVID-19 who are at risk of progression, VV116 is similarly effective to Paxlovid with respect to the time to sustained clinical recovery, with fewer safety concerns[8].
Many companies have jumped on the bandwagon to find better 3C-like protease inhibitors, given this strategy has been the most successful approach so far and seems to be variant-proof, with the protease being highly conserved across current variants. In November 2022, Japanese pharmaceutical company Shionogi announced its protease inhibitor Xocova (ensitrelvir) had received emergency regulatory approval in Japan. Shionogi is now conducting a global phase III trial in non-hospitalised SARS-CoV-2-infected patients[9]. Pardes Biosciences, based in California, is also conducting a phase II trial of its oral protease inhibitor PBI-0451 in non-hospitalised symptomatic adults, with results expected in mid-2023[10].
Enanta Pharmaceuticals has its own protease inhibitor, EDP-235, currently in phase II trials in non-hospitalised adults, which it hopes to report on in the first half of 2023[11]. Luly says the company is aiming for a “best in class” molecule that is not only potent but can be dosed daily and given without the need for ritonavir boosting. “We achieved that preclinical profile,” he says, with “good blood levels at the 24-hour time point”. Luly explains that the drug more efficiently penetrates lung tissue, where the virus replicates, than nirmatrelvir, which he hopes will provide an advantage and could solve the rebound problem.
Others are pursuing second-generation nucleoside analogues, an approach taken from the development of the first HIV/AIDS drugs more than 40 years ago. Sommadossi, whose company Atea is developing an oral nucleoside analogue bemnifosbuvir (AT-527) with Roche, says this strategy is a good bet for COVID-19 since the targeted RNA polymerase enzyme RdRp is “probably the most conserved” of all the viral enzymes, being used for both replication and transcription. As well as this, Sommadossi says, Atea’s drug has an additional ability to directly inhibit nucleotidyltransferase (NiRAN), the enzyme that catalyses the transfer of a nucleotide residue from one compound to another, potentially creating a higher barrier to resistance.
No other antiviral against SARS-CoV-2 we are aware of combines these two modes of action
Christiane Schaffitzel, cryo-electron microscopy Specialist at the University of Bristol
In October 2021, Atea and Roche reported that AT-527 had not shown significant reduction in the amount of SARS-CoV-2 virus in patients with mild or moderate COVID-19 compared with placebo in their phase II trial, but had done so in high-risk patients with underlying health conditions[12]. Since then, they have started a phase III trial in this group, with the hope of providing an option without the drug–drug interaction problems seen with Paxlovid for this cohort[13]. They expect to report new data in early 2024.
Other companies are pursuing completely new mechanisms for fighting SARS-CoV-2. One comes from start-up Halo Therapeutics in Bristol, based on a discovery by Christiane Schaffitzel, cryo-electron microscopy Specialist at the University of Bristol. In 2020, she found a pocket in the SARS-CoV-2 spike protein that binds the essential fatty acid linoleic acid (LA) and, in doing so, stabilises it into a locked conformation, stopping the virus binding to the ACE2 receptor and entering the cell[14]. Schaffitzel,’s group also found that flooding cells with LA shut down the synthesis of phospholipases, stopping any virus particles from building organelles in which to replicate.
“No other antiviral against SARS-CoV-2 we are aware of combines these two modes of action,” says Schaffitzel. The pocket is present in all SARS-Cov-2 variants and many other beta-coronaviruses, including MERS-CoV — the deadly coronavirus that appeared in 2012 — but not others, such as those that cause the common cold, which Schaffitzel suggests may mean it is a feature of severe disease.
Halo is planning clinical trials for an LA nasal spray in 2023. “We are convinced a spray application into the nose is the best route to target the virus, catch it early and forestall disease progression,” explains Schaffitzel. Other antivirals that can be delivered nasally are also being investigated, including the compound aprotinin, previously developed to treat influenza. Aprotinin is a broad-spectrum protease inhibitor that is able to prevent the processes by which the spike protein is cleaved to allow the virus to fuse with the cell membrane. A phase III trial in Spain showed the drug could be used to bolster other treatments for hospitalised patients[15].
One of the major problems with current COVID-19 antivirals is they tend to be most effective when given very early in the course of the infection because they impact viral replication. They have less impact on the later inflammatory stages of the disease, which is usually when serious illness develops. In fact, says Radu Pislariu, chief executive of Laurent Pharma in Montreal, Canada, “we are not dealing with one disease here … it’s three diseases actually”, with the viral phase being followed by pulmonary inflammation and, for some people, a hyper-inflammatory phase, where inflammation is out of control and often fatal.
This kind of ‘host-directed’ approach has the added advantage of not being sensitive to the SARS-CoV-2 variant
Radu Pislariu, chief executive of Laurent Pharma
Laurent is taking a combined approach with its antiviral LAU-7b and trying to tackle both the virus and the inflammation. LAU-7b is derived from the small molecule drug fenretinide, developed by Laurent to treat cystic fibrosis, a disease that can also lead to out-of-control inflammation owing to cell membrane lipid imbalances. “The manifestation of the diseases is quite similar,” says Pislariu. So the company has developed an oral drug candidate that inhibits a desaturase enzyme, leading to a rigid membrane with more saturated fatty acids that is difficult for the virus to penetrate. It also activates pathways that resolve inflammation by preventing the hyper-inflammatory responses seen in severely ill COVID-19 patients. This kind of ‘host-directed’ approach has the added advantage of not being sensitive to the SARS-CoV-2 variant: “That’s a way to avoid resistance,” says Pislariu.
Overall, topline results from Laurent’s phase II study of 232 patients hospitalised with moderate, severe or critical COVID-19 disease, announced in September 2021, show it failed to improve the proportion of subjects alive and free of respiratory failure compared with placebo[16]. But, compared with placebo, LAU-7b did find efficacy in a pre-specified subgroup of 148 moderate-to-severe COVID-19 patients, with a 100% reduction in risk of progression to mechanical ventilation or death after 60 days (with 4 in the placebo arm dying)[16]. Based on this, the company launched a phase III trial in the United States and Canada in February 2022, which is ongoing[17].
Alisdair Macdonald
Another approach is to use drug combinations, rather than relying on a monotherapy, a strategy already common in treating HIV/AIDS. “I’m pretty sure that this is what’s going to be the future, especially for immunocompromised patients,” says Sommadossi, “One drug may not be sufficient.” Atea is planning a parallel trial to assess a combination of its novel nucleoside analogue and a second-generation protease inhibitor, which would target two key viral enzymes and could avoid the current problem of drug–drug interactions with Paxlovid. Other researchers have been investigating combinations that synergistically inhibit SARS-CoV-2, such as a recent study combining antiviral nucleoside analogues with anti-inflammatory pyrimidine biosynthesis inhibitors — drugs used to treat multiple sclerosis[18].
Keep exploring
Luly says it is important to keep exploring new ways to “go after” SARS-CoV-2, “because, at the end of the day, you never know which mechanism will be the most important long term”. Michaelis agrees; one possibility for the future is to look at cell metabolism, which is shifted by the virus, and find molecules that can inhibit these changes. Another is to directly target the molecules that mediate inflammation, such as interferons.
What appears certain is that COVID-19 is not going away, and other viruses are hot on its heels, with fears of a ‘triple-demic’ this winter of COVID-19, influenza and respiratory syncytial virus. Although a second generation of antivirals is on the way, there is no time for complacency; we need to keep going, says Pislariu, “so we are not caught off guard the next time by another virus or another pandemic”.
- This article was amended on 16 January 2023 to clarify that remdesivir, GS-5245 and VV116 have different chemical structures and on 19 January to correct the number of participants in the subgroup analysis of Laurent Pharma’s phase II LAU-7b trial
- 1Treatments for Highest Risk Non-Hospitalised Patients (Adults and Children) with COVID-19. Central Alerting System. 2022.https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=103218 (accessed 11 Jan 2023).
- 2NICE recommends 3 treatments for COVID-19 in draft guidance. NICE. 2022.https://www.nice.org.uk/news/article/nice-recommends-3-treatments-for-covid-19-in-draft-guidance (accessed 11 Jan 2023).
- 3Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. Merck. 2021.https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/ (accessed 11 Jan 2023).
- 4Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022;386:509–20. doi:10.1056/nejmoa2116044
- 5Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. The Lancet. 2022. doi:10.1016/s0140-6736(22)02597-1
- 6Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 2022;386:1397–408. doi:10.1056/nejmoa2118542
- 7Gilead Sciences. Study Evaluating GS-5245 in Participants With COVID-19 Who Have a High Risk of Developing Serious or Severe Illness. ClinicalTrials.gov. 2022.https://www.clinicaltrials.gov/ct2/show/NCT05603143 (accessed 11 Jan 2023).
- 8Cao Z, Gao W, Bao H, et al. VV116 versus Nirmatrelvir–Ritonavir for Oral Treatment of Covid-19. N Engl J Med. 2022. doi:10.1056/nejmoa2208822
- 9University of Minnesota. Strategies and Treatments for Respiratory Infections &Amp; Viral Emergencies (STRIVE): Shionogi Protease Inhibitor. ClinicalTrials.gov. 2022.https://clinicaltrials.gov/ct2/show/NCT05605093 (accessed 11 Jan 2023).
- 10Pardes Biosciences. PBI-0451 Phase 2 Study in Nonhospitalized Symptomatic Adults With COVID-19. ClinicalTrials.gov. 2022.https://clinicaltrials.gov/ct2/show/NCT05543707?term=PBI-0451&draw=2&rank=1 (accessed 11 Jan 2023).
- 11Enanta Pharmaceuticals. A Study to Evaluate EDP-235 in Non-hospitalized Adults With COVID-19 (SPRINT). ClinicalTrials.gov. 2022.https://clinicaltrials.gov/ct2/show/NCT05616728?term=EDP-235&draw=2&rank=3 (accessed 11 Jan 2023).
- 12Atea Pharmaceuticals . Atea Pharmaceuticals Provides Update and Topline Results for Phase 2 MOONSONG Trial Evaluating AT-527 in the Outpatient Setting. Atea Pharmaceuticals . 2021.https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-provides-update-and-topline-results-phase-2/ (accessed 11 Jan 2023).
- 13Atea Pharmaceuticals. SUNRISE-3: Efficacy and Safety of Bemnifosbuvir in High-Risk Outpatients With COVID-19. ClinicalTrials.gov. 2022.https://clinicaltrials.gov/ct2/show/NCT05629962?term=AT-527&draw=3&rank=18 (accessed 11 Jan 2023).
- 14Toelzer C, Gupta K, Yadav SKN, et al. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science. 2020;370:725–30. doi:10.1126/science.abd3255
- 15Bojkova D, Bechtel M, McLaughlin K-M, et al. Aprotinin Inhibits SARS-CoV-2 Replication. Cells. 2020;9:2377. doi:10.3390/cells9112377
- 16Laurent Pharma. Laurent Pharmaceuticals Announces Topline Results from its Phase 2 RESOLUTION Clinical Trial of LAU-7b for the Treatment of COVID-19. Laurent Pharma. 2021.https://laurentpharma.com/laurent-pharmaceuticals-announces-topline-results-from-its-phase-2-resolution-clinical-trial-of-lau-7b-for-the-treatment-of-covid-19/ (accessed 11 Jan 2023).
- 17Schultz DC, Johnson RM, Ayyanathan K, et al. Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2. Nature. 2022;604:134–40. doi:10.1038/s41586-022-04482-x
- 18Laurent Pharmaceuticals. Study of LAU-7b for the Treatment of COVID-19 Disease in Adults (RESOLUTION). ClinicalTrials.gov. 2020.https://www.clinicaltrials.gov/ct2/show/NCT04417257 (accessed 11 Jan 2023).



