Andrea Ucini
When deciding to prescribe a medicine to alleviate depression, doctors and patients face a fraught process of trial and error. After the initial clinical evaluation, a doctor’s first choice antidepressant has a 50–50 chance of working, and this usually takes several weeks to find out[1].
This fuzzy standard of care comes with real risks. Patients may have to manage side effects, such as weight gain or agitation, while remaining uncertain about whether the drug will quell their depression symptoms. This may result in some patients discontinuing with their prescription and lead them to distrust their doctors. Worse still, when faced with the possibility of unremitting depression, it may even lead some patients to suicide.
“We know that it is an inexact process. Physicians admit that, patients experience it, and so we need more personalised prescribing strategies,” says Chad Bousman, a pharmacogenomics researcher at the University of Calgary, Canada.
Depression affects 4.5% of people in the UK[2]. Rates of depressive symptoms soared during the COVID-19 pandemic, peaking at 21% of adults in Great Britain during early 2021, more than double the rate observed before the pandemic[3].
Trial and error
Fortunately, there is a range of antidepressant medicines available, including selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants.
If a patient bounces back and forth between drugs trying to get stabilised, they do worse later on
John Papastergiou, pharmacist and pharmacy owner in Toronto, Canada
This variety compounds the difficulty of finding an effective medicine for a particular person, however, and it can take multiple tries before getting it right.
“If a patient bounces back and forth between drugs trying to get stabilised, they do worse later on,” says John Papastergiou, a pharmacist and pharmacy owner in Toronto, Canada.
“But when patients get on the right drugs faster, they do better down the line.”
Those who do not get relief face a long and difficult road. Long durations of unremitted depression are associated with a worse prognosis, including poorer clinical outcomes, greater side effects, higher medical costs and decreased work productivity[4][5–7].
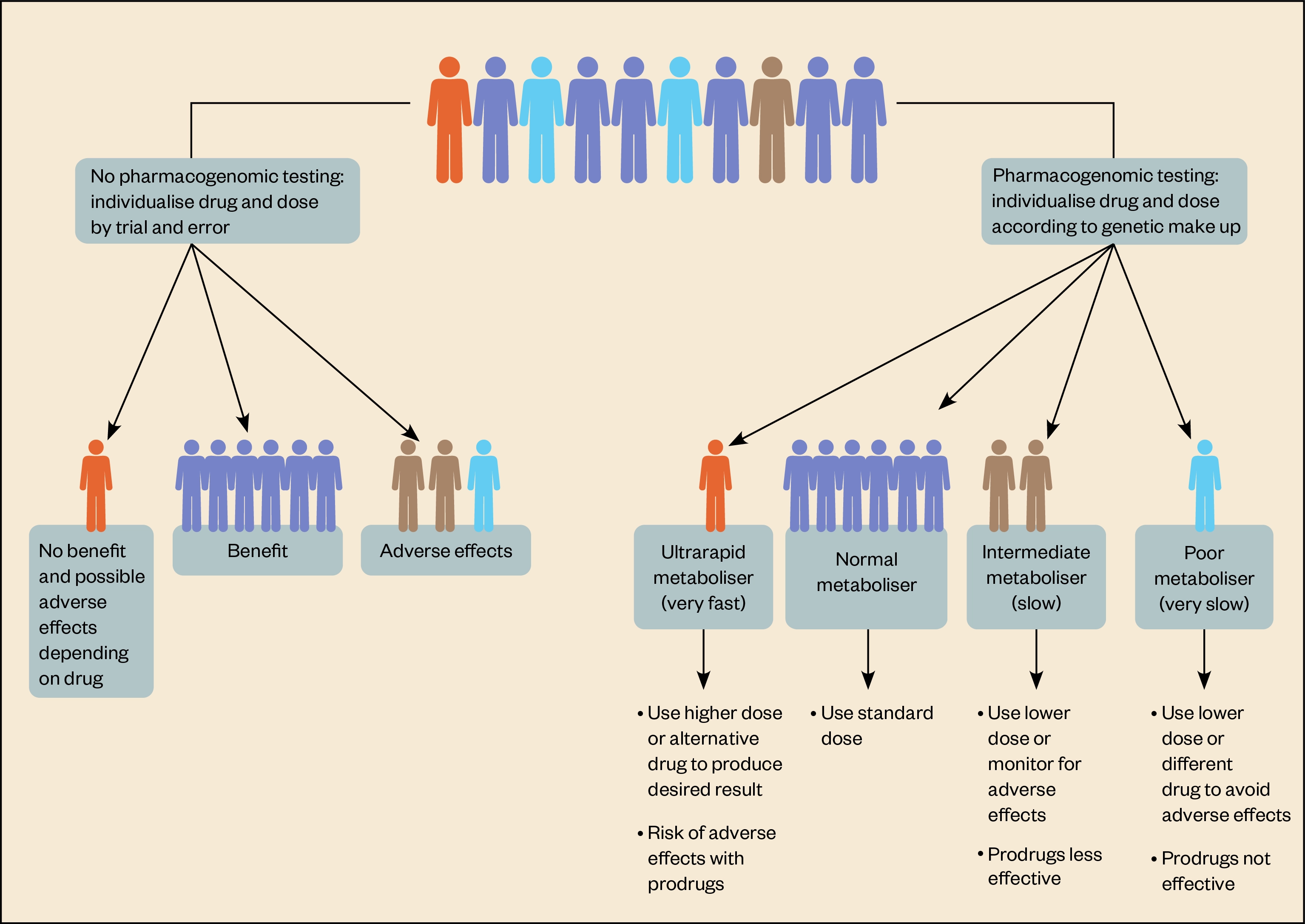
Pharmacogenomic information could help avoid these consequences by expediting these treatment choices. Individuals differ in their genetic makeup and this could translate into differences in how the body breaks down a drug or how the drug acts on the body (see Box). Knowing a person’s genotype across different genes related to drug metabolism or drug targets could optimise drug efficacy and avoid adverse effects. This possibility is being studied for a variety of drugs and conditions, including studies focusing on depression.
Box: What do pharmacogenomic tests look at?
Pharmacogenomic tests look for genetic variants that are associated with a variable response to specific medicines, either by affecting how the body metabolises these medicines or how it responds to them.
Most drugs are metabolised in humans by 12 enzymes belonging to the cytochrome P450 (CYP) family. Two of these — CYP2D6 and CYP2C19 — are key for breaking down antidepressants[8]. The genes encoding CYP2D6 and CYP2C19 are highly variable, with many single nucleotide polymorphisms (SNPs) within them; researchers have documented nearly 150 alleles of CYP2D6 and over 30 in CYP2C19.
A person’s genotype across these genes can indicate how well their body will metabolise a drug and, as a result, potentially predict drug response. For example, those with genotypes reflecting ultra-rapid metabolism of a drug might need higher than usual doses to benefit.
Likewise, those identified as poor metabolisers will have higher than usual drug levels, which could make them prone to side effects and adverse drug reactions (see Figure). However, most people fall somewhere in the middle.
Allele frequencies vary in different populations, which means that the prevalence of ultra-rapid metabolisers or poor metabolisers can vary according to genetic ancestry. For example, the Finnish population has a relatively high frequency of a CYP2D6 genotype associated with ultra-rapid metabolism[9].
Most tests also include genes encoding presumed targets of a drug, such as SLC6A4, which encodes the serotonin transporter, but evidence for their usefulness in predicting drug response is lacking.
Interest in pharmacogenomic testing may be outpacing the evidence base. Several pharmacogenomic tests are commercially available, which differ in the genetic variants they evaluate. Meanwhile, researchers are still uncovering gene–drug interactions and establishing standards for the ones worth paying attention to.
Some randomised clinical trials of pharmacogenomic tests show some promise for prescribing in depression, and larger trials are on the way. At the same time, healthcare systems need to put into place infrastructure that will allow pharmacogenomic testing to become a routine part of prescribing — something pharmacists will be instrumental in.
The trick will be to ride the enthusiasm for pharmacogenomics while adhering to rigorous standards.
“This is just the beginning and so it’s exciting,” says Bousman, who is also a member of the Clinical Pharmacogenetics Implementation Consortium (CPIC), where he is involved in creating practice guidelines about which pharmacogenomic results should guide antidepressant prescribing.
“But we need to keep these commercial companies [that manufacture and market the tests] from imploding the whole field because they want to implement pharmacogenomics faster than it should be,” he warns.
Mixed evidence
Current pharmacogenomic tests detect genetic variation within a limited panel of genes, including cytochrome P450 (CYP) enzymes and a handful of others. Unlike a whole genome sequence, the restricted search gives results quickly and cheaply. Tests differ in the details of which variants are included — some tests, such as GeneSight, focus on genetic variation relevant to psychiatric drugs, whereas others, such as Pillcheck, are generalists that examine genetic variation related to a broad range of medicines for a variety of conditions.
The variability in allele frequencies across different populations, combined with the different genes included in the different panels, can make the usefulness of pharmacogenomic testing tricky to pin down.
Randomised clinical trials are making headway into this, but the current evidence for antidepressant prescribing shows mixed results. In 2019, results from a 1,167-participant trial — GUIDED (‘Genomics used to improve depression decisions’) — in the United States showed that patients who had inadequately responded to previous medicines showed a higher rate of symptom improvement and remission after pharmacogenomic-guided prescribing[10]. Results from a smaller trial in Canada, published in 2022, found similar benefits, but they fell short of statistical significance[11].
To make sense of the available trials, Bousman published a meta-analysis in 2019, combining results from five randomised clinical trials, including the GUIDED trial. This found that those receiving pharmacogenomic-guided prescriptions were 71% more likely to have remission of their symptoms than those receiving standard care without pharmacogenomic testing[12].
“The trial data are leaning in the direction of this being a useful tool,” says Bousman. He notes two potential sources of bias: companies making the panels also funded the studies; and trial participants were predominately of European genetic background, which makes the generalisability of the findings uncertain.
Larger studies may help address these issues. In the EU, a multi-centre study across seven countries — PREPARE (‘PREemptive Pharmacogenomic Testing for Preventing Adverse Drug Reactions’) — is examining whether pharmacogenomic testing of 44 variants in 12 genes reduces adverse drug reactions in nearly 7,000 study participants taking a wide range of drugs, including antidepressants[13]. In the United States, a large trial — PRIME Care — is focused on pharmacogenomic testing for antidepressant prescribing in 2,000 veterans with depression[14].
Pharmacodynamic search
Much less is known about genetic variation that affects drug pharmacodynamics — that is, how the drug exerts its effect on the body. Though current pharmacogenomic tests examine candidate genes suspected to be involved in drug action, such as SLC6A4 or HTR2A — which encode a serotonin transporter and a serotonin receptor, respectively — these and other genes’ relationships to drug response is unproven.
“I think it is pure 100% marketing when those genes are included [in the panels],” Bousman says. “It has nothing to do with science.”
We need to have a much broader view that engages with the more complex and undiscovered question of how the body responds to a given drug
Andrew McIntosh, a psychiatrist at the University of Edinburgh
However, the search space for these genes is vast. Instead of guessing which genes might be involved, the Psychiatric Genomics Consortium (PGC) has opted for an unbiased scan of the entire genome to look for common variants that make a difference for antidepressant response. A first crack at this did not detect any genome-wide specific associations with treatment response in more than 5,000 participants, but it did find that part of treatment response was indeed heritable[15].
“We need to have a much broader view that engages with the more complex and undiscovered question of how the body responds to a given drug,” says Andrew McIntosh, a psychiatrist at the University of Edinburgh, who also co-chairs the PGC’s Major Depressive Disorder Working Group.
Future studies will have to be much larger to detect a meaningful signal, and the PGC — which is already more than 200 investigators strong — will need to incorporate data from others willing to share.
In the meantime, McIntosh says that he would not advocate using current CYP gene-dominated panels to guide his drug decisions for his patients with depression. Standard practice involves starting a person at a low dose of a drug and carefully monitoring them for side effects and efficacy, which can indirectly reveal whether someone metabolises the drug in an unusual manner.
“It’s kind of incorporating the genotype of the patient, but not explicitly,” he says.
“Genotyping for side effect risk or treatment response may, one day, provide meaningful information,” he adds.
“I think the evidence isn’t yet compelling, but it’s important that people are conducting these studies.”
Real world preparation
The evidence is already sufficient for some health care insurers. In particular, results from a 2021 study led by Papastergiou prompted Green Shield Canada, which funded the study, to pay for genetic tests. Operated out of three community pharmacies in Toronto, Canada, the study recruited more than 200 outpatients with depression or anxiety, administered a Pillcheck test to a randomised subset and then used validated questionnaires to measure symptoms of depression or anxiety at baseline and at different follow-up times. By six months, those who had received pharmacogenomic testing reported more symptom relief than those who did not[16].
Pharmacists can play a big role here, because they understand drugs, how they work, and because they see the patient regularly
John Papastergiou, a pharmacist and pharmacy owner in Toronto, Canada
“This was a real-world study that showed the value of this type of testing,” says Papastergiou.
It also showed that pharmacists could be instrumental in implementing pharmacogenomics: pharmacists conducted the tests, then conveyed the results to prescribers, who made the final decisions.
“Pharmacists can play a big role here, because they understand drugs, how they work, and because they see the patient regularly,” he adds.
Cost could deter pharmacogenomic testing from becoming routine, since tests currently range from CAD$100–350 (£65–225) apiece. However, a test may help save on healthcare costs down the line if it reduces adverse drug reactions, drug switching and the time spent in hospital. A 2017 study, funded by a test manufacturer, found that pharmacogenomic testing for mental illness resulted in medicines cost savings of nearly US$4,000 (£3,360) per year[17].
Even as insurers come on board, experts are working to establish which gene-drug interactions are actionable. The Pharmacogenomics Knowledgebase is an online database that lists gene-drug pairs with sufficient evidence, as determined by the CPIC, the Dutch Pharmacogenetics Working Group and the Canadian Pharmacogenomics Network for Drug Safety. This kind of database helps users interpret the results returned from tests[18].
Healthcare systems in the UK are beginning to explore how to integrate pharmacogenomic testing into routine care. In March 2022, the Royal College of Physicians and the British Pharmacological Society published a report that outlines steps needed to make pharmacogenomic-based prescribing a reality for all within the NHS[19].
Establishing the needed infrastructure will be challenging. In contrast to other countries, such as the Netherlands, the UK has no centralised, standard prescribing system[20].
“In the UK, every single hospital, GP practice and community pharmacy has a completely separate [prescribing] system,” says Victoria Rollinson, a clinical pharmacist at Cheshire and Wirral Partnership NHS Foundation Trust. She was involved in recruiting patients at the University of Liverpool for the PREPARE study.
The NHS Genomics Medicine Service is currently developing infrastructure plans for pharmacogenomic testing[21]. Decisions will need to be made about who to genotype, how it will be done, where it will be done and how to return the results. Ideally, a person’s test results could be kept in an electronic health record, with potential drug–gene interactions flagged by a computer. This would have to be updated as new evidence comes in.
Despite these challenges, interest is high among both patients and clinicians in mental health settings, says Adam Jameson, a mental health pharmacist and researcher at the Bradford District Care NHS Foundation Trust.
In 2021, Jameson and colleagues published a review of studies regarding attitudes towards pharmacogenomic testing for mental health[22]. While the review identified barriers such as the cost of testing or lack of infrastructure as challenges to its implementation in mental healthcare settings, it also held up enabling factors, such as shared interest among patients and clinicians for pharmacogenetic testing, and an expectation that it would become more routine in the future.
Some clinicians also suggested the benefits of pharmacogenomic-guided prescribing could extend beyond a drug’s action in the brain if it improved the rapport between clinicians and patients.
For depression, a solid patient-clinician relationship is not simply nice to have, but it can be fundamental to a person regaining their mental health.
“This could be very important because the relationship between the patient and the prescriber is really very key in psychiatry,” Jameson says.
- 1Undurraga J, Baldessarini RJ. Randomized, Placebo-Controlled Trials of Antidepressants for Acute Major Depression: Thirty-Year Meta-Analytic Review. Neuropsychopharmacol. 2011;37:851–64. doi:10.1038/npp.2011.306
- 2Mental Health. Our World in Data. 2021.https://ourworldindata.org/mental-health#depression (accessed 22 Jun 2021).
- 3Coronavirus and depression in adults, Great Britain: January to March 2021. Office for National Statistics. 2021.https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/articles/coronavirusanddepressioninadultsgreatbritain/januarytomarch2021 (accessed 22 Jun 2022).
- 4Ghio L, Gotelli S, Marcenaro M, et al. Duration of untreated illness and outcomes in unipolar depression: A systematic review and meta-analysis. Journal of Affective Disorders. 2014;152–154:45–51. doi:10.1016/j.jad.2013.10.002
- 5Ghio L, Gotelli S, Cervetti A, et al. Duration of untreated depression influences clinical outcomes and disability. Journal of Affective Disorders. 2015;175:224–8. doi:10.1016/j.jad.2015.01.014
- 6Mrazek DA, Hornberger JC, Altar CA, et al. A Review of the Clinical, Economic, and Societal Burden of Treatment-Resistant Depression: 1996–2013. PS. 2014;65:977–87. doi:10.1176/appi.ps.201300059
- 7Trivedi MH, Morris DW, Wisniewski SR, et al. Increase in Work Productivity of Depressed Individuals With Improvement in Depressive Symptom Severity. AJP. 2013;170:633–41. doi:10.1176/appi.ajp.2012.12020250
- 8Rollinson V, Turner R, Pirmohamed M. Pharmacogenomics for Primary Care: An Overview. Genes. 2020;11:1337. doi:10.3390/genes11111337
- 9Pietarinen P, Tornio A, Niemi M. High Frequency ofCYP2D6Ultrarapid Metabolizer Genotype in the Finnish Population. Basic Clin Pharmacol Toxicol. 2016;119:291–6. doi:10.1111/bcpt.12590
- 10Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. Journal of Psychiatric Research. 2019;111:59–67. doi:10.1016/j.jpsychires.2019.01.003
- 11Tiwari AK, Zai CC, Altar CA, et al. Clinical utility of combinatorial pharmacogenomic testing in depression: A Canadian patient- and rater-blinded, randomized, controlled trial. Transl Psychiatry. 2022;12. doi:10.1038/s41398-022-01847-8
- 12Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20:37–47. doi:10.2217/pgs-2018-0142
- 13PREemptive Pharmacogenomic Testing for Preventing Adverse Drug REactions (PREPARE). ClinicalTrials.gov. 2019.https://clinicaltrials.gov/ct2/show/NCT03093818 (accessed 22 Jun 2022).
- 14PRIME Care (PRecision Medicine In MEntal Health Care) (PRIME Care). https://clinicaltrials.gov/ct2/show/NCT03170362. 2022.https://clinicaltrials.gov/ct2/show/NCT03170362 (accessed 22 Jun 2022).
- 15Pain O, Hodgson K, Trubetskoy V, et al. Identifying the Common Genetic Basis of Antidepressant Response. Biological Psychiatry Global Open Science. 2022;2:115–26. doi:10.1016/j.bpsgos.2021.07.008
- 16Papastergiou J, Quilty LC, Li W, et al. Pharmacogenomics guided versus standard antidepressant treatment in a community pharmacy setting: A randomized controlled trial. Clin Transl Sci. 2021;14:1359–68. doi:10.1111/cts.12986
- 17Brown LC, Lorenz RA, Li J, et al. Economic Utility: Combinatorial Pharmacogenomics and Medication Cost Savings for Mental Health Care in a Primary Care Setting. Clinical Therapeutics. 2017;39:592-602.e1. doi:10.1016/j.clinthera.2017.01.022
- 18Bousman CA, Zierhut H, Müller DJ. Navigating the Labyrinth of Pharmacogenetic Testing: A Guide to Test Selection. Clin. Pharmacol. Ther. 2019;106:309–12. doi:10.1002/cpt.1432
- 19Royal College of Physicians, British Pharmacological Society. Personalised prescribing: using pharmacogenomics to improve patient outcomes. Royal College of Physicians 2022. https://www.rcplondon.ac.uk/projects/outputs/personalised-prescribing-using-pharmacogenomics-improve-patient-outcomes (accessed 22 Jun 2022).
- 20Thornley T, Esquivel B, Wright DJ, et al. Implementation of a Pharmacogenomic Testing Service through Community Pharmacy in the Netherlands: Results from an Early Service Evaluation. Pharmacy. 2021;9:38. doi:10.3390/pharmacy9010038
- 21NHS Genomic Medicine Service. NHS. https://www.england.nhs.uk/genomics/nhs-genomic-med-service/ (accessed 22 Jun 2022).
- 22Jameson A, Fylan B, Bristow GC, et al. What Are the Barriers and Enablers to the Implementation of Pharmacogenetic Testing in Mental Health Care Settings? Front. Genet. 2021;12. doi:10.3389/fgene.2021.740216