
Kinga Howard (Unsplash)
Introduction
Phosphodiesterase 5 receptor (PDE5) inhibitors have had a significant impact on the treatment of erectile dysfunction (ED), with the introduction of drugs like sildenafil citrate (Viagra®), tadalafil (Cialis®), and vardenafil (Levitra®). In 1998, Pfizer launched the first PDE5 inhibitor therapy (sildenafil citrate) to treat erectile dysfunction (ED) under the brand name Viagra®[1]. This drug revolutionised the treatment of the condition, being the first clinically tested oral medication for this problem. Despite being developed as a cardiovascular drug (for angina), it soon became apparent this drug was effective at restoring erectile health and it became a mainstay of treatment[2], largely replacing other therapies, including intra-cavernosal injection (Caverject®) and mechanical methods, such as vacuum pumps. Following on from this, other drugs in the class were developed with tadalafil (Cialis®) and vardenafil (Levitra®) as two notable examples arriving on the market in 2003[3,4].
As confidence in prescribing grew in this group of drugs and it became apparent that the PDE5 inhibitor class had a favourable benefit/risk profile, responsibility for providing ED care moved from the specialist to the general practitioner. Over time, in the UK, responsibility for supply was expanded to specially trained pharmacists under a Patient Group Directive (PGD)[5].
In 2014, New Zealand became the first country to allow supply directly from a pharmacy without prescription[6]. This was followed in 2016 by the availability of sildenafil in Poland[7] and the approval, in 2018, of Viagra Connect® as a Pharmacy (P) medicine in the UK. Norway and Ireland also allowed the supply of Viagra® without prescription. Many generic manufacturers have followed Viagra’s® lead, and, in addition, tadalafil was approved as a product from pharmacies in Poland in 2022[8]. The MHRA has now approved Cialis Together 10mg tablets (tadalafil) as a Pharmacy (P) medicine, available without prescription.
This article will look at what this means for both patients and pharmacists in an increasingly sophisticated Pharmacy ED treatment landscape. Consequently, this article seeks to clarify the similarities and differences between the main PDE5 inhibitor molecules, and to provide information on the P medicine options that are now available for UK men seeking treatment.
PDE5 inhibitors common characteristics and mode of action
Phosphodiesterase type 5 inhibitors (PDE5 inhibitors) share common characteristics and a similar mode of action. These medications work by blocking the action of the enzyme PDE5, which is responsible for breaking down cyclic GMP (cGMP) in the smooth muscle cells lining the blood vessels.
By inhibiting PDE5, these drugs promote the accumulation of cGMP, leading to vasodilation in the smooth muscle cells of the blood vessels. This dilation specifically occurs in the corpora cavernosa of the penis, facilitating an erection when sexual stimulation is present. Therefore, PDE5 inhibitors are primarily used in the treatment of erectile dysfunction (ED)[9].
In addition to their effect on the penile blood vessels, PDE5 is also present in the smooth muscle of the arterioles within the lungs. This has led to the approval of sildenafil and tadalafil for the treatment of pulmonary hypertension[10]. Tadalafil has also been licensed for the treatment of benign prostatic hyperplasia[11]. While there is ongoing research into the cardiovascular benefits of PDE5 inhibitors, this topic will not be discussed further in this article.
It is important to note that non-prescription treatments for ED are limited to this specific indication only. The information mentioned above is provided for reference purposes and to assist pharmacists in directing patients accordingly when they seek advice at the pharmacy.
Contraindications
There are certain contraindications to consider when using PDE5 inhibitors. If patients are taking soluble guanylate cyclase stimulators (such as riociguat), or nitrate medications such as isosorbide mononitrate or isosorbide dinitrate, PDE5 inhibitors should not be taken within 24 hours (or 48 hours with tadalafil). Concurrent use of these medications can cause a significant drop in blood pressure, which is rare but a potentially dangerous consequence. Care must also be exercised when used in combination with alpha blockers such as doxazosin[12].
This group of drugs are also contraindicated in patients with previous nonarteritic anterior ischaemic optic neuropathy(NAION) and hereditary eye diseases. Despite initial concerns of adverse cardiovascular events in patients prescribed PDE5 inhibitors, several long-term studies have established the safety of these drugs in both healthy patients and patients with cardiovascular risk factors[9].
Adverse effects
Although all PDE5 inhibitors share the same mechanism of action, each agent has different pharmacokinetics and pharmacodynamics, which affect how quickly they act, how long their effects will last, and their side effects. Notably, although this class of drugs preferentially inhibit PDE5, the degree to which they also inhibit other phosphodiesterases influences their side effect profile.
For example, sildenafil also inhibits PDE6, which is present in the retina of the eye; this reaction is thought to be responsible for the temporary visual changes that some patients using sildenafil experience. Similarly, tadalafil also inhibits PDE11, which is present in the prostate, although no effects on fertility have been reported. Although agents more selective for PDE5 were in development, these trials have been suspended, likely due to the saturation of the market with the introduction of agents with broad cardiovascular benefits, such as SGLT2 inhibitors and endothelin receptor antagonists[9].
Overall, PDE5 inhibitors are generally well tolerated, and side effects are usually mild to moderate and of short duration. The occurrence of side effects, or adverse drug reactions (ADRs), with PDE5 inhibitors depends on the dose and type of agent. Headache is a very common ADR with sildenafil, occurring in >10% of patients, and is slightly less common with tadalafil occurring in 1–10% of patients. Back pain and muscle aches are more common in patients taking tadalafil. Other common ADRs include dizziness, flushing, dyspepsia, nasal congestion or rhinitis[12,13]. Side effects tend to be transient, and if problematic, alternative therapies are available.
In 2007, the U.S. Food and Drug Administration (FDA) announced that a warning about possible sudden hearing loss would be added to drug labels of PDE5 inhibitors[14]. This is a rare phenomenon and patients should be advised to seek urgent assistance if this occurs.
Drug interactions
PDE5 inhibitors are primarily metabolized by the cytochrome P450 enzyme system, particularly CYP3A4. Therefore, there is a potential for drug interactions with other drugs that inhibit or induce CYP3A4. This includes drugs like HIV protease inhibitors, ketoconazole, and itraconazole. However, co-administration has not been linked to changes in the safety or efficacy of either agent.
Combining PDE5 inhibitors with vasodilators, such as nitroglycerin, isosorbide mononitrate and dinitrate, is contraindicated due to the potential for life-threatening hypotension, which may rarely occur[12].
The main agents marketed globally are sildenafil, tadalafil, vardenafil and avanafil . It is important that patients do not use any of these agents concurrently and should seek medical advice if they want to switch to an alternative PDE5 inhibitor.
Mechanism of action
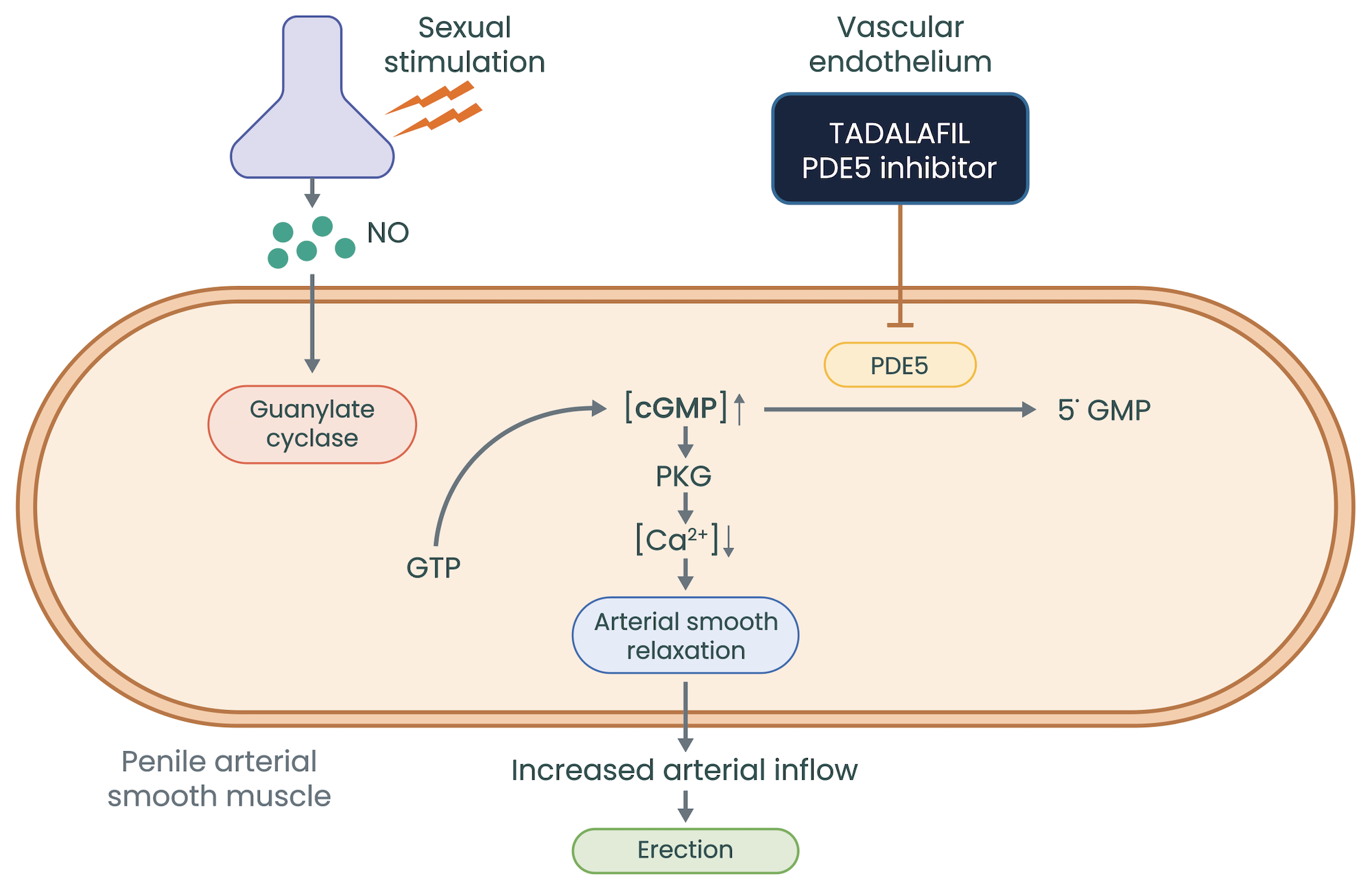
Adapted from Cruz-Burgos M et al. 2021
Vasodilatation occurs when nitric oxide (NO) is released in response to sexual stimulation, by vascular endothelial cells, causing relaxation of vascular smooth muscle cells. Nitric oxide activates soluble guanylate cyclase, which converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP), the main effector of the system. For example, in the penis, NO release at high levels from endothelial cells and penile nerves during sexual stimulation leads to relaxation of the smooth vasculature of the corpus cavernosum, causing vasocongestion and a sustained erection, whilst sexually stimulated[1].
PDE5 inhibitors prolong the action of cGMP by inhibiting its degradation by the enzyme PDE5, which is found throughout the body. In the penis, PDE5 inhibitors potentiate the effects of cGMP to prolong erections and increase sexual satisfaction; however, PDE5 inhibitors do not cause erections without sexual stimulation (see Table 2[12,13,15–17]).
Sildenafil was discovered by Pfizer in 1989 and was initially being developed for the treatment of cardiovascular issues[2]. In the early trials it soon became apparent it was having other effects on the subjects and consequently the indication and trial program were radically altered.
Sildenafil is a relatively short acting molecule and from a pharmacokinetic perspective, has a duration of action of 4–5 hours, with a half-life of around 4 hours. Sildenafil is rapidly absorbed and is best taken on an empty stomach as it has a pH sensitive absorption profile. Sildenafil’s efficacy is dose dependent, with around 82% of sufferers responding to the 100mg dose and 74% to the 50mg dose, which is the recommended starting dose[13]. There is a 25mg product for special populations or men with toleration issues.
The most common side effects are transient, such as headache, facial flushing, and visual disturbance, manifesting as blue flashing before the eyes in some patients. Overall, the benefit-risk profile of the drug is favourable and those who should not use the product are well characterised. These include (not an exhaustive list) nitrate users, patients not fit enough to have sex and patients with Peyronie’s disease[13].
Tadalafil was discovered in the late 1990s by Eli Lilly in Indianapolis and was approved and launched in the US and other markets in 2003. The onset of action of tadalafil is typically around half an hour after taking the tablet, and the duration is up to 36 hours. The half-life of the drug is around 17.5 hours. Like sildenafil, common side effects include flushing, nasal congestion, and dyspepsia. Again, caution is advised in those with active cardiovascular disease and is not recommended in people taking nitrate drugs, such as glyceryl trinitrate.
Tadalafil can be taken with or after food without affecting the absorption; it is also important to note the drug does not work without sexual stimulation and is efficacious for a window of about 36 hours[12]. This does not mean the patient will experience a constant erection during that period unless there is stimulation. In addition, if the man is in the post ejaculation refractory phase, he will be unlikely to get a subsequent erection until he has recovered.
What pharmacists and patients need to know about the currently available non-prescription ED products?
Both sildenafil and tadalafil are licensed for the treatment of erectile dysfunction (ED). ED is classed as the inability to obtain and maintain an erection satisfactory for sexual intercourse. In the UK, sildenafil 50mg tablets and tadalafil 10mg tablets are available without a prescription from pharmacies, as an on-demand treatment for ED.
If patients raise issues like overactive bladder or prostate issues, or even concerns about heart health, they should be directed to their medical practitioner for more specific advice on these issues. If patients find the non-prescription doses of PDE5 inhibitor are not addressing their ED problems or are taking the drug more frequently than suggested by the label, again they should be directed to their medical practitioner for further advice and assessment.
For most men, a single PDE5 inhibitor on-demand non-prescription dose will work the first or second time they try it. For men who have not been able to have sexual intercourse for some time, it can take additional attempts to obtain maximum benefit. If after several attempts (up to 8) on different dosing occasions, patients are still not able to achieve a penile erection sufficient for satisfactory sexual activity, they should be advised to consult their doctor.
Lastly, all men enquiring about the use of a non-prescription ED medication should be encouraged to go to their medical practitioner for a check up of the underlying causes. This being irrespective of whether they obtain a non-prescription supply of a PDE5 inhibitor product or not.
In terms of pharmacist advice, there are several things to consider when recommending which PDE5 inhibitor to be used. The main considerations are:
- Timing of the dose relative to the point of need. Sildenafil should be taken approximately 1 hour before sexual activity. Tadalafil should be taken at least 30 minutes before anticipated sexual activity. As such, it is important a man understands he may have to wait before the drug has reached a therapeutic blood level, and in the case of sildenafil, if a high-fat meal has been consumed, the onset of action may be delayed.
- It is also important to point out that sildenafil will be available in the system for 4 to 5 hours after ingestion, whereas for tadalafil, the therapeutic effect is seen up to 36 hours after dosing. What is very important is that the man understands that the drug does not lead to an erection without sexual stimulation and will not (in all but the rarest cases) lead to a permanent erection. Anyone experiencing this very rare phenomena should seek urgent medical assistance.
- Given the different half-lives of the 2 drugs, (sildenafil 4 hours and tadalafil 17.5 respectively) it may be important to understand a patient’s attitude to planning and spontaneity. For some men, using a shorter acting drug may be preferable, but for men who may need more time and confidence the drug is in the system for a longer period of time, then tadalafil may be preferable.
- In addition, it is also important to remind patients, that following sexual intercourse and ejaculation, the normal recovery or refractory period will need to pass before a man can perform sexually again. This will vary from person to person and is also age related. This class of drugs are not considered performance enhancers and should not be viewed as such, rather as drugs that restore normal function in men with a compromised erectile process.
- Another factor to consider is whether an individual can take this class of drugs with food. While not an absolute for sildenafil, studies have shown taking the product with a high-fat meal can slow down the absorption of the drug. The solubility of the drug is pH dependent and decreases as pH rises, so some patients may experience a slower onset of action and a lower resultant blood level with a heavy meal. Tadalafil absorption, on the other hand, is not affected by the presence of food.
- It is also important to counsel patients about side effects (see above) and reassure the sufferer about the transient nature of these effects. Finally, patients should be questioned about any contraindications and advised to see their medical practitioner for follow up; this is also an opportunity to provide some basic healthy living advice.
Conclusion
The community pharmacy has become an important part of the healthcare system in many countries and the availability of ED medications provides a widening scope for provision of efficacious treatments. The pharmacy offers convenience and familiarity to patients, and medicines for ED are provided in a responsible and thoughtful way.
With increasing numbers of non-prescription ED products, it is important pharmacists understand the differences with each treatment offering; thus, being able to provide both expert advice and tailoring treatment to the patient need is extremely important. It also gives the pharmacist an opportunity to engage this group of patients and provide an informed choice about the options available.
This article highlights the key similarities and differences of the available non-prescription medicines and aims to address common questions that arise when choosing a medication for ED. By understanding the mechanisms of action, duration of action, side effects, and factors to consider when recommending a specific medication, pharmacists can better assist patients in making informed decisions about their treatment.
Further information about Cialis® Together, including essential supply information, adverse reactions, precautions, contraindications, and method of use can be found at: https://www.medicines.org.uk/emc/
Cialis® Together is available in packs of 4 and 8 tablets. RRP (excluding VAT): 4 tablets pack £18.33; 8 tablets pack £31.66.
MAT-XU-2305374 v1.0 December 2023
- 1Drug Approval Package: Viagra (sildenafil citrate). US Food and Drug Administration. 1998. https://www.accessdata.fda.gov/drugsatfda_docs/NDA/98/viagra/viagra_toc.cfm (accessed April 2024)
- 2Goldstein I, Burnett AL, Rosen RC, et al. The Serendipitous Story of Sildenafil: An Unexpected Oral Therapy for Erectile Dysfunction. Sexual Medicine Reviews. 2019;7:115–28. https://doi.org/10.1016/j.sxmr.2018.06.005
- 3Cialis FDA Approval History. Drugs.com. https://www.drugs.com/history/cialis.html (accessed April 2024)
- 4Levitra becomes Viagra’s first US challenger. Pharmafile. 2003. https://pharmafile.com/news/levitra-becomes-viagras-first-us-challenger/ (accessed April 2024)
- 5Proposed Changes to NHS Availability of Erectile Dysfunction Treatments Changing Prescribing: Restrictions for Sildenafil. Department of Health. 2014. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/274600/2014_01_23_Final_Consultation_Document.pdf (accessed April 2024)
- 6Move over Viagra: Silvasta from Douglas Pharmaceuticals is chasing share of erectile dysfunction market. Idealog. 2015. https://idealog.co.nz/venture/2015/05/move-over-viagra-silvasta-douglas-pharmaceuticals-chasing-share-erectile-dysfunction-market (accessed April 2024)
- 7OTC Bulletin. Citeline. 2016. https://hbw.citeline.com/-/media/supporting-documents/otc-bulletin-pdfs/2016/otc-bulletin-463.pdf (accessed April 2024)
- 8Ridley D. Poland’s Adamed Secures Tadalafil Rx-To-OTC Switch In World First. Citeline. 2022. https://hbw.citeline.com/RS152274/Polands-Adamed-Secures-Tadalafil-Rx-To-OTC-Switch-In-World-First (accessed April 2024)
- 9Tzoumas N, Farrah TE, Dhaun N, et al. Established and emerging therapeutic uses of PDE type 5 inhibitors in cardiovascular disease. British J Pharmacology. 2020;177:5467–88. https://doi.org/10.1111/bph.14920
- 10Goldstein I, Lue TF, Padma-Nathan H, et al. Oral Sildenafil in the Treatment of Erectile Dysfunction. N Engl J Med. 1998;338:1397–404. https://doi.org/10.1056/nejm199805143382001
- 11WANG Y, BAO Y, LIU J, et al. Tadalafil 5 mg Once Daily Improves Lower Urinary Tract Symptoms and Erectile Dysfunction: A Systematic Review and Meta‐analysis. LUTS. 2016;10:84–92. https://doi.org/10.1111/luts.12144
- 12Cialis Together 10mg tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/14803/smpc (accessed April 2024)
- 13Viagra Connect 50 mg film-coated tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/8725/smpc (accessed April 2024)
- 14Rao A, Thwaini A, Ahmed H, et al. The phosphodiesterase inhibitors and non-arteritic anterior ischaemic optic neuropathy: increased vigilance is necessary. BJU Int. 2007;100:3–4.
- 15Phosphodiesterase-5 (PDE-5) inhibitors. National Institute for Health and Care Excellence. https://cks.nice.org.uk/topics/erectile-dysfunction/prescribing-information/phosphodiesterase-5-pde-5-inhibitors/ (accessed April 2024)
- 16Vardenafil 20 mg tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/9658/smpc (accessed April 2024)
- 17Spedra 100 mg tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/7421/smpc (accessed April 2024)
You may also be interested in

UK Clinical Pharmacy Association launches pharmacogenomics guidance
Health news round-up: cardiovascular disease, women’s health and the potential for new sepsis treatment
