Coneyl Jay / Science Photo Library
In 2010, researchers carried out a study in which a group of patients with Parkinson’s disease were given a pill and told that they had a 75% chance of receiving levodopa, a drug that increases dopamine levels in the brain, and a 25% chance of receiving placebo. Brain imaging revealed that dopamine release occurred and tests showed motor function improved. But the researchers did not give the patients levodopa. In fact, they all received a placebo. Extraordinarily, simply telling the patients that they were likely to receive the drug led to dopamine release[1]
.
Ted Kaptchuk, a placebo researcher at Harvard Medical School and director of the world’s first interdisciplinary research centre for placebo studies, the Program in Placebo Studies and the Therapeutic Encounter (PiPS) at Beth Israel Deaconess Medical Centre, Boston, Massachusetts, likens healthcare to a ‘drama’, with the placebo effect a central player. “That drama involves cues and environmental and contextual factors such as [medical] paraphernalia, pills, symbols and the interaction with the doctor, which involves a lot of emotional support, attention, empathy and compassion,” he explains. “All those things merge, triggering brain mechanisms of expectation and potentially contribut[ing] to a placebo effect.”
Placebo responses appear to arise from several brain mechanisms and neurochemical pathways. Conscious and subconscious psychological mechanisms, such as expectation and conditioning, both play a crucial role. And it is now becoming evident that a network of genes may influence whether and how people respond to a placebo — a finding that could potentially transform patient care and clinical trial design.
Damien Finniss, a researcher at the University of Sydney’s Pain Management Research Institute, acknowledges the complexity of the placebo effect, and suggests that we should think about it in terms of many different phenomena, rather than one. He adds that you do not have to give a placebo to obtain a placebo effect; it is part and parcel of routine clinical care.
The term ‘placebo’ is traditionally used to describe inert agents such as sugar pills or saline injections (pure placebos). But it is also employed for agents that contain an active ingredient that is not recommended for the condition being treated, for example, giving antibiotics for a viral infection (impure placebos).
The placebo effect, while powerful, does have limitations. Yes, it can produce clinical benefits in conditions in which the central nervous system is involved, such as pain or anxiety, but it cannot stop disease processes, such as tumour growth.
Neurobiological mechanisms
The best understood example of the placebo effect is in the reduction of pain. Research suggests that this effect results from distinct neurobiological changes in pain pathways, rather than ‘report bias’ — wholesale alterations in the subjective perception of pain2. For example, blood flow measurements with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have revealed that placebo analgesic responses are accompanied by reduced neural activity in specific pain-processing areas of the brain and spinal cord[2]
.
In 1978, Jon Levine and colleagues from the University of California at San Francisco reported a study in which patients were given either a placebo or naloxone, which has no pain relief effect but blocks opioid receptors, after dental surgery. Those who were given naloxone reported significantly more pain than those on placebo, suggesting that endogenously released opioids play a part in pain reduction from the placebo effect[3]
. Subsequent studies confirmed that placebo analgesia is, at least partly, mediated by the endogenous opioidergic system through the activation of the μ-subtype of opioid receptors[2]
.
Placebo responses may also be able to tap into dopamine-mediated reward pathways. In a landmark American study using a special type of PET scanning that enables researchers to look specifically at dopamine in the brain, University of Michigan researchers demonstrated that when pain is anticipated, placebo administration is accompanied by dopamine release in a ‘reward area’: the nucleus accumbens[4]
. The more participants expected a benefit from the placebo, the more they experienced pain relief.
In the same study, analyses of fMRI data showed that dopamine release in the nucleus accumbens also occurred during anticipation of a monetary reward, and that this was proportional to placebo-induced dopamine release[4]
. It is possible that the more excitable your reward pathways are, the more you are likely to enjoy a placebo effect.
More recently, a team at the University of Turin, Italy, investigated whether cannabinoids can be involved in placebo analgesic effects. They gave participants a non-steroidal anti-inflammatory drug (NSAID), before a pain challenge on consecutive days. Once the participants were pharmacologically conditioned to expect pain relief, they replaced the drug with a placebo. The resultant placebo response could be reversed by the cannabinoid 1 receptor antagonist, rimonabant, suggesting that endogenous release of cannabinoids may contribute to some instances of placebo analgesia[5]
.
Expectation and conditioning
The placebo effect is not limited to pain relief. Research on motor control disorders, particularly Parkinson’s disease, has shown that it can work there, too. Significant improvements in Parkinson’s disease symptoms, and concomitant increases in dopaminergic neurotransmission in the area of the brain affected by the disorder, the striatum, have been observed after patients were given a placebo they thought was an anti-Parkinsonian agent[6]
.
Furthermore, as shown by the 2010 study in which Parkinson’s disease sufferers were told what their likelihood was of getting levodopa, patients’ expectation of a reward — a clinical improvement in this case — affects dopamine release in the striatum. In the PET study, led by Jon Stoessl, who directs the Pacific Parkinson’s Research Centre in Vancouver, Canada, patients were told that they had either a 25%, 50%, 75% or 100% chance of receiving the active drug. Although they were all given a placebo, only those who believed they had a 75% probability of getting levodopa showed significant dopamine release and symptom improvement[1]
. This is in keeping with studies on conditioned learning, which show dopamine release when reward is likely but not certain. Whether a patient had previously had a good response to levodopa treatment was also a major contributor to dopamine release, which is consistent with pre-conditioning[1]
.
“Repeated association between a real drug and a response can lead to a conditioning, or learning, process, whereby the act of taking a drug, even a placebo, can lead to a biological response,” explains Finniss. It is now generally accepted that conditioning and expectation are both involved in the placebo effect.
Repeated association between a real drug and a response can lead to a conditioning, or learning, process, whereby the act of taking a drug, even a placebo, can lead to a biological response
Many cues can feed into the placebo effect, both conscious and subconscious. Even the size, colour and branding of a placebo pill can affect whether or not a person will experience a response. But not all contributing factors have been identified. Similarly, it is not clear what can influence the magnitude of the placebo response.
A recent systematic review suggests that there is no difference in the magnitude of clinical benefit in response to more intensive placebo treatments; for example, those that take longer or are more invasive[7]
. However, another analysis suggests that patients could experience larger benefits from physical placebo interventions, such as sham acupuncture, rather than from pharmacological ones[8]
. The perceived cost of the placebo treatment also appears to have an impact on the clinical benefit obtained[9]
.
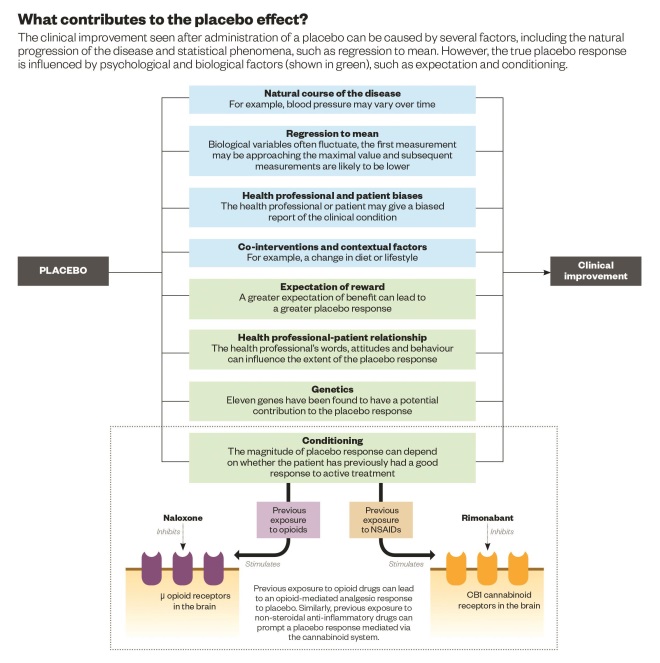
Source: Physiological Reviews 2013;93:1207–1246.
The placebome
It is not only external cues that can act as predictors of placebo response. Individual genetic variation is emerging as an important factor. The theory was first explored in 2012 by Harvard Medical School researchers led by Kathryn Hall, who randomised patients with irritable bowel syndrome to no treatment, placebo acupuncture alone, or placebo acupuncture enhanced by supportive interaction with the treatment provider. All patients underwent genetic screening[10]
.
Hall’s team found that patients in the enhanced treatment arm who had two copies of a variant of the gene encoding the catechol-O-methyltransferase (COMT) enzyme in which a valine was substituted for methionine at position 158 (Val158Met), had the strongest response to placebo treatment. COMT is one of the enzymes involved in the regulation of dopamine levels. The findings point to variations in the COMT gene as a potential predictor of placebo response.
The research has fuelled the idea that there may be a set of genes — the placebome — that are responsible for differences in the way people respond to placebos.
Those genes may be many and varied. In a 2015 study[11]
, Hall and colleagues reviewed relevant research from the past decade and concluded that, besides the dopamine system, genetic variations that influence other major neurotransmitters, such as the opioid, cannabinoid and serotonin systems, could mediate the placebo effect.
“We found 11 genes … involved in different neural pathways,” says Kaptchuk, a co-author of the study. He suggests that, “There may be genetic signatures that … play a part in determining who is more, or less, likely to respond to a placebo.”
There may be genetic signatures that … play a part in determining who is more, or less, likely to respond to a placebo
It is probably not as simple as a set of genes determining whether or not someone is a placebo responder. Kaptchuk says that the genes they have identified are not a guarantee of placebo response; they need to be activated by environmental, contextual and other factors. And, since these factors will likely be different for different situations, even if someone responds once, it does not mean that they always will. Additionally, notes Kaptchuk, different genes or groups of genes are likely to affect placebo responses in different medical conditions, so someone may be a placebo responder for one illness but not for another.
Trial design
If confirmed, the recent findings by Hall and colleagues could have implications for placebo-controlled clinical trials designed to test new drugs, particularly small studies.
“The beauty of large randomised trials is that people with different genotypes tend to be evenly balanced across arms,” says Tim Lahey, a clinical ethicist at Dartmouth’s Geisel School of Medicine, in Hanover, New Hampshire. Because of this, the results of the study overall are unlikely to be affected by differences in placebo response profiles, he explains. “The problem is that for smaller studies, if you have an unintentional imbalance in either side of the comparison, you might get differences between the groups that are explained entirely by genetics and not at all by whether patients receive the drug or placebo.”
Matthew Wynia, director of the Center for Bioethics and Humanities at the University of Colorado in Aurora, also worries about the effect of genetics. “If more placebo-sensitive individuals are in the drug arm than in the placebo arm … the drug looks like it’s better than it actually is.” On the other hand, he adds, if more placebo responders are in the control arm, the apparent effectiveness of the drug could be diminished.
If more placebo-sensitive individuals are in the drug arm than in the placebo arm … the drug looks like it’s better than it actually is
Genetic screening of trial participants may be useful to ensure the reliability of results. However, this is not always appropriate. “Current drug testing [is moving] towards ‘comparative effectiveness studies’ that do not include a placebo arm”, says Paul Enck, a medical psychologist and director of research at University Hospital Tübingen, in Germany, referring to studies in which one active drug is compared with another.
In trials that do include a placebo group, another approach is to add a control group that receives no treatment, in addition to the placebo arm, to separate the effect of the drug from that of the placebo. This can also enable researchers to assess the magnitude of the placebo effect.
The placebome could have implications well beyond clinical trial design. Blogging for the British Medical Journal, Geoffrey Modest, of Boston University School of Medicine, Massachusetts, notes that identifying placebo responders through genetic screening could lead to effective non-medical interventions, avoiding the side effects associated with drug treatment[12]
.
Modest also points out that many drugs act through the same neural pathways involved in the placebo effect. Consequently, it is important to determine whether the drug and placebo effects augment or compete with each other in any given situation.
All this means that it is possible that treating placebo responders and non-responders with the same drug could have different results. Genetic screening could help predict and address these possibilities.
Kaptchuk believes that “the fact that we found 11 genes with [a] potential contribution to the placebo effect” suggests that researchers have only uncovered the tip of an iceberg. “It’s really a first step in what is probably a more elaborate and complex scene.”
References
[1] Lidstone SC, Schulzer M, Dinelle K et al. Effects of expectation on placebo-induced release in Parkinson’s disease. Journal of the American Medical Association 2010;67:857–865.
[2] Schedlowski M, Enck P, Rief W, Bingel U. Neuro-behavioral mechanisms of placebo and nocebo responses: implications for clinical trials and clinical practice. Pharmacological Reviews 2015;67:697–730.
[3] Levine JD, Gordon NC, Fields HL. The mechanisms of placebo analgesia. The Lancet 1978;2:654–657.
[4] Scott JD, Stohler CS, Egnatuk CM et al. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 2007;55:325–336.
[5] Benedetti F, Amanzio M, Rosato R, Blanchard C. Nonopioid placebo analgesia is mediated by CB1cannabinoid receptors. Nature Medicine 2011;17:1228–1230.
[6] de la Fuente-Fernández R, Ruth TJ, Sossi V et al. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science 2001;293:1164–1166.
[7] Fässler M, Meissner K, Kleijnen J et al. A systematic review found no difference in effect between more and less intensive placebo interventions. Journal of Clinical Epidemiology 2015;68:442–451.
[8] Linde K, Niemann K, Meissner K. Are sham acupuncture interventions more effective than (other) placebos? A re-analysis of data from the Cochrane review on placebo effects. Forschende Komplementärmedizin 2010;17:259–264.
[9] Espay AJ, Norris MM, Eliassen JC et al. Placebo effect of medication cost in Parkinson’s disease: a randomized double-blind study. Neurology 2015;84:794–802.
[10] Hall KT, Lembo AJ, Kirsh I et al. Catechol-O-methyltransferase val58met polymorphism predicts placebo effect in irritable syndrome. PLoS ONE 2012;7: e48135.
[11] Hall KT, Loscalzo J, Kaptchuk TJ. Genetics and the placebo effect: the placebome. Trends in Molecular Medicine 2015;21:285–294.
[12] Modest G. Placebo genetics and the placebome. British Medical Journal Blogs. Weblog. Online 7 May 2015.